E8656
Heat-Labile Enterotoxin, B subunit (LTB) from E. coli
recombinant, expressed in Pichia pastoris, >90% (SDS-PAGE), lyophilized powder
Synonym(s):
LTB
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352200
NACRES:
NA.77
Recommended Products
recombinant
expressed in Pichia pastoris
Quality Level
Assay
>90% (SDS-PAGE)
form
lyophilized powder
shipped in
wet ice
storage temp.
2-8°C
Application
Heat-Labile Enterotoxin, B subunit (LTB) from E. coli has been used in enzyme-linked immunosorbent assay (ELISA).
Biochem/physiol Actions
Enterotoxigenic Escherichia coli causes diarrhea through its heat-labile enterotoxin (LT). The LT is a periplasmic protein composed of one A subunit (LTA, 27 kDa) and five non-covalently associated B subunits (LTB, 11.6 kDa each) forming a ring-like pentamer. LTB has high affinity towards the toxin receptor ganglioside GM1, a glycosphingolipid found ubiquitously on the surface of mammalian cells. Ganglioside GM1 facilitates the delivery of the A subunit to the cytosol of the target cell resulting in persistent synthesis of cAMP and subsequently diarrhea. This characteristic makes LTB a good label for microglial cells (due to the enrichment of ganglioside GM1 on their cell surface). In addition, studies made mostly with animal models demonstrated that recombinant LTB could stimulate strong serum and mucosal immune responses against LT. Other studies have indicated that LTB could be used as a potent mucosal adjuvant.
Features and Benefits
This compound is a featured product for Cyclic Nucleotide research. Click here to discover more featured Cyclic Nucleotide products. Learn more about bioactive small molecules for other areas of research at sigma.com/discover-bsm.
Physical form
Lyophilized from a solution containing Heat-Labile Enterotoxin, B subunit in 0.05 M Tris buffer, pH 7.5, 0.2 M NaCl, 3 mM NaN3, and 1 mM sodium EDTA.
Analysis Note
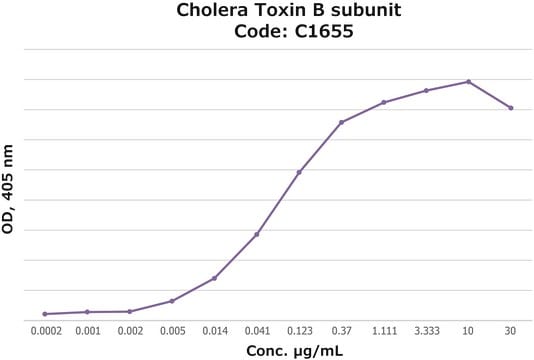
The activity is measured by ELISA using ganglioside GM1-coated plates (Cat. No. G7641). LTB at various concentrations is incubated on the ganglioside GM1 coated wells, followed by anti-cholera toxin antibody (Cat. No. C3062), and peroxidase-labeled goat anti-rabbit IgG (Cat.No. A0545) as the secondary antibody. Binding saturation of 50% may be achieved with = 0.12 μg/mL of LTB.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Aquatic Chronic 3
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Sandra Scheiblhofer et al.
Vaccine, 39(32), 4399-4403 (2021-07-07)
The skin represents an attractive target tissue for vaccination against respiratory viruses such as SARS-CoV-2. Laser-facilitated epicutaneous immunization (EPI) has been established as a novel technology to overcome the skin barrier, which combines efficient delivery via micropores with an inherent
Plastid expression of a double-pentameric vaccine candidate containing human papillomavirus-16 L1 antigen fused with LTB as adjuvant: transplastomic plants show pleiotropic phenotypes
Waheed MT, et al.
Plant Biotechnology Journal, 9(6) (2011)
Safety and immunogenicity of escalating dosages of a single oral administration of peru-15 pCTB, a candidate live, attenuated vaccine against enterotoxigenic Escherichia coli and Vibrio cholerae
Chen WH, et al.
Clinical and Vaccine Immunology : CVI, 22(1) (2015)
Preformulation Characterization and Stability Assessments of Secretory IgA Monoclonal Antibodies as Potential Candidates for Passive Immunization by Oral Administration
Hu Y, et al.
Journal of Pharmaceutical Sciences, 109(1) (2020)
Yue Hu et al.
Journal of pharmaceutical sciences, 109(1), 407-421 (2019-08-02)
Enterotoxigenic Escherichia coli (ETEC) is a major cause of diarrheal disease among children in developing countries, and there are no licensed vaccines to protect against ETEC. Passive immunization by oral delivery of ETEC-specific secretory IgAs (sIgAs) could potentially provide an
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service