539164
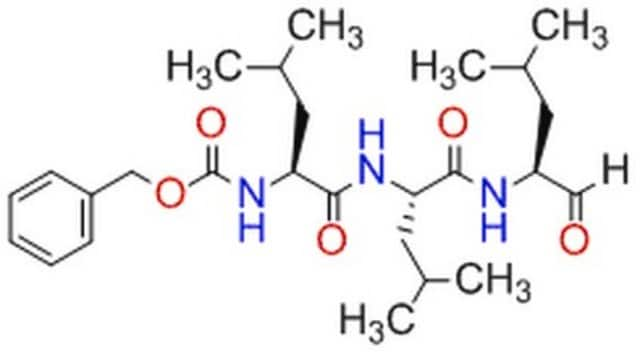
Proteasome Inhibitor Set I
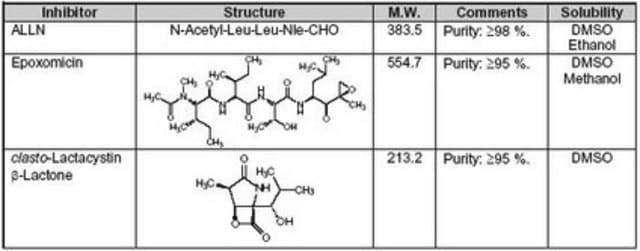
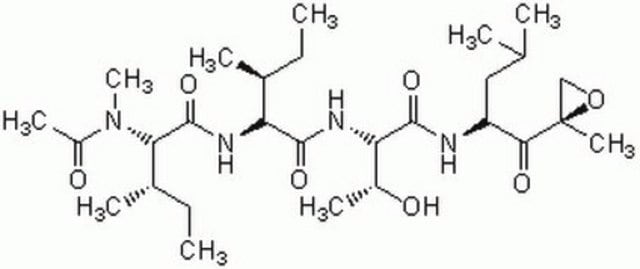
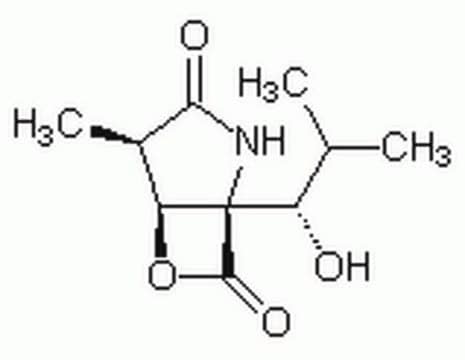
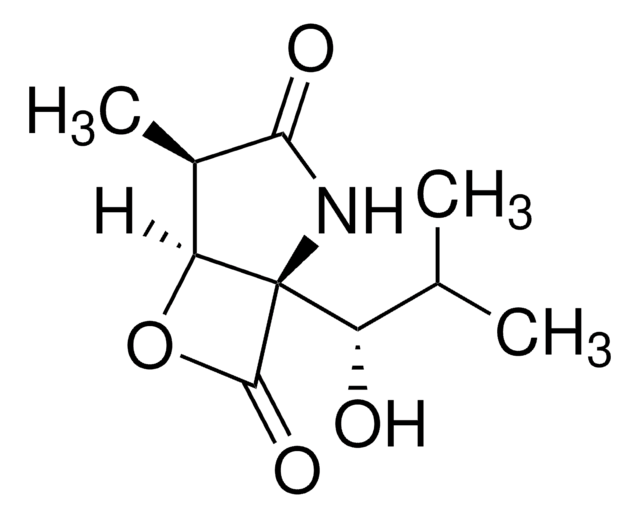
The Proteasome Inhibitor Set I controls the biological activity of Proteasome. This small molecule/inhibitor is primarily used for Protease Inhibitors applications.
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352200
NACRES:
NA.77
Recommended Products
Quality Level
form
solid
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
shipped in
ambient
storage temp.
−20°C
General description
Proteasomes are large multi-subunit protease complexes, localized in the nucleus and cytosol, which selectively degrade intracellular proteins. They play a major role in the degradation of many proteins involved in cell cycling, proliferation, and apoptosis. A vast majority of short-lived proteins are degraded by the ubiquitin-proteasome pathway. A protein marked for degradation is covalently attached to multiple molecules of ubiquitin (Ubq), a highly conserved 76-amino acid (8.6 kDa) protein, which escorts it for rapid hydrolysis to the multi-component enzymatic complex known as the 26S proteasome. The proteolytic core of this complex, the 20S proteasome, contains multiple peptidase activities and functions as the catalytic machine.
The ubiquitin-proteasome pathway plays a major role in the breakdown of abnormal proteins that result from oxidative stress and mutations that might otherwise disrupt normal cellular homeostasis. The reactive oxygen species can promote partial unfolding of the proteins, exposing its hydrophobic domains to proteolytic enzymes of the 20S complex. Rapid degradation of defective enzymes, as seen in diseases caused by metabolic abnormalities, also occurs in the proteasome. The Ubq-proteasome pathway has also been implicated in several forms of malignancy, in the pathogenesis of several genetic diseases, and in the pathology of muscle wasting. It is also involved in the destruction of proteins that participate in cell cycle progression, transcription control, signal transduction, and metabolic regulation.
The ubiquitin-proteasome pathway plays a major role in the breakdown of abnormal proteins that result from oxidative stress and mutations that might otherwise disrupt normal cellular homeostasis. The reactive oxygen species can promote partial unfolding of the proteins, exposing its hydrophobic domains to proteolytic enzymes of the 20S complex. Rapid degradation of defective enzymes, as seen in diseases caused by metabolic abnormalities, also occurs in the proteasome. The Ubq-proteasome pathway has also been implicated in several forms of malignancy, in the pathogenesis of several genetic diseases, and in the pathology of muscle wasting. It is also involved in the destruction of proteins that participate in cell cycle progression, transcription control, signal transduction, and metabolic regulation.
Biochem/physiol Actions
Primary Target
MPC
MPC
Secondary Target
NF-κB activity
NF-κB activity
Warning
Toxicity: Standard Handling (A)
Other Notes
Grimm, L.M., et al. 1996. EMBO J.15, 3835.
Griscavage, J.M., et al. 1996. Proc. Natl. Acad. Sci. USA93, 3308.
Lee, D.H., and Goldberg, A.L. 1996. J. Biol. Chem.271, 27280.
Oda, K., et al. 1996. Biochem. Biophys. Res. Commun.219, 800.
Fenteany, G., et al. 1995. Science268, 726.
Traenckner, E.B., et al. 1994. EMBO J.13, 5433.
Griscavage, J.M., et al. 1996. Proc. Natl. Acad. Sci. USA93, 3308.
Lee, D.H., and Goldberg, A.L. 1996. J. Biol. Chem.271, 27280.
Oda, K., et al. 1996. Biochem. Biophys. Res. Commun.219, 800.
Fenteany, G., et al. 1995. Science268, 726.
Traenckner, E.B., et al. 1994. EMBO J.13, 5433.
Legal Information
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service