All Photos(1)
About This Item
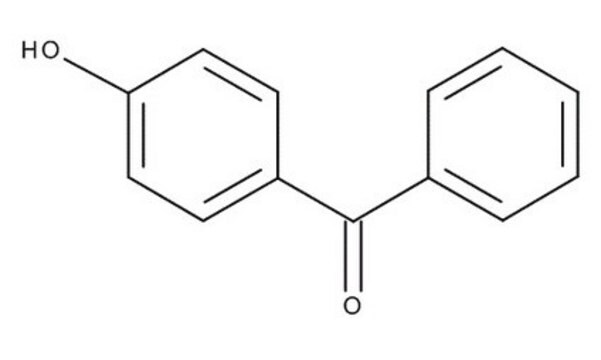
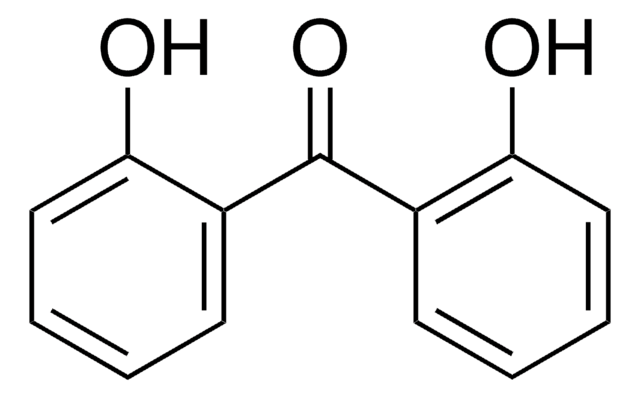
Linear Formula:
HOC6H4COC6H5
CAS Number:
Molecular Weight:
198.22
Beilstein:
777776
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
98%
mp
132-135 °C (lit.)
SMILES string
Oc1ccc(cc1)C(=O)c2ccccc2
InChI
1S/C13H10O2/c14-12-8-6-11(7-9-12)13(15)10-4-2-1-3-5-10/h1-9,14H
InChI key
NPFYZDNDJHZQKY-UHFFFAOYSA-N
Gene Information
rat ... Ar(24208)
Looking for similar products? Visit Product Comparison Guide
General description
4-Hydroxybenzophenone is characterized by its ability to absorb UV radiation and convert it into lower-energy radiation. This property makes it an effective photostabilizer. Its molecular structure features two aromatic rings connected by a carbonyl group, allowing for strong π-π stacking interactions, which enhance its stability and efficacy in polymer matrices. Incorporating 4-Hydroxybenzophenone into polymer matrices increases the longevity and performance of materials used in outdoor applications, such as coatings and plastics.
Application
4-Hydroxybenzophenone can be used:
- As a photoinitiator to synthesize fluorescent triblock copolymer micelles for targeted release of cancer drugs and intracellular bioimaging. It facilitates controlled release, improving therapeutic efficacy while minimizing side effects.
- As a photoacid catalyst for ring opening polymerization of lactones to prepare biodegradable polyesters.
- As a precursor to synthesize functional copolymer for fabrication of nanostructured transparent metal electrodes for flexible optoelectronic devices.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3 - STOT RE 2
Target Organs
Liver,Kidney
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Daniel Molins-Delgado et al.
The Science of the total environment, 601-602, 975-986 (2017-06-06)
The increased use of beauty and other daily use products, in particular those containing UV filters (UV-Fs) and benzotriazoles, results in their introduction in significant amounts into the aquatic environment. In this study, we aim to assess the occurrence and
Aneesh Karkhanis et al.
Biochemical pharmacology, 146, 188-198 (2017-09-30)
Cardiac enzymes such as cytochrome P450 2J2 (CYP2J2) metabolize arachidonic acid (AA) to cardioprotective epoxyeicosatrienoic acids (EETs), which in turn are metabolized by soluble epoxide hydrolase (sEH) to dihydroxyeicosatrienoic acids (DHETs). As EETs and less potent DHETs exhibit cardioprotective and
Francesco Barsotti et al.
Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology, 16(4), 527-538 (2017-01-20)
The photophysics and photochemistry of 4-hydroxybenzophenone (4HOBP) are interesting because they can give some insight into the behavior of humic material. Here we show that 4HOBP has a number of fluorescence peaks: (i) an intense one at excitation/emission wavelengths Ex/Em
Benzophenone metabolism. I. Isolation of p-hydroxybenzophenone from rat urine.
A W Stocklinski et al.
Life sciences, 26(5), 365-369 (1980-02-04)
Adela Jing Li et al.
Journal of hazardous materials, 337, 115-125 (2017-05-17)
Ethyl-4-aminobenzoate (Et-PABA) is currently used as a substitute for 4-aminobenzoate (PABA) in sunscreens and anesthetic ointments. Despite its widespread use and hydrophilicity, Et-PABA has never been found in environmental waters. This study, probed the occurrence of Et-PABA in both seawater
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service