A78608
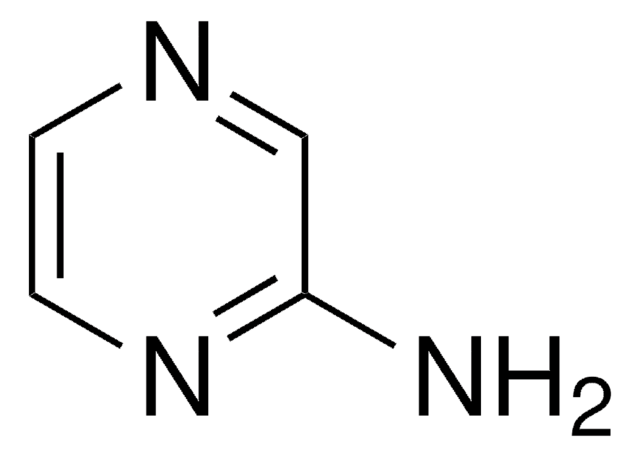
2-Aminopyrimidine
97%
Synonym(s):
2-Pyrimidinamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H5N3
CAS Number:
Molecular Weight:
95.10
Beilstein:
107014
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
powder
mp
122-126 °C (lit.)
SMILES string
Nc1ncccn1
InChI
1S/C4H5N3/c5-4-6-2-1-3-7-4/h1-3H,(H2,5,6,7)
InChI key
LJXQPZWIHJMPQQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
D S Ermolat'ev et al.
Molecular diversity, 15(2), 491-496 (2010-08-27)
An efficient microwave-assisted one-pot two-step protocol was developed for the construction of disubstituted 2-amino-1H-imidazoles. This process involves the sequential formation of 2,3-dihydro-2-hydroxyimidazo[1,2-a]pyrimidinium salts from readily available 2-aminopyrimidines and α-bromoketones, followed by cleavage of the pyrimidine ring with hydrazine.
Rogier A Smits et al.
Drug discovery today, 14(15-16), 745-753 (2009-05-30)
The search for new and potent histamine H4 receptor ligands is leading to a steadily increasing number of scientific publications and patent applications. Several interesting and structurally diverse compounds have been found, but fierce IP competition for a preferred 2-aminopyrimidine
S A Abdel-Latif et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 67(3-4), 950-957 (2006-11-07)
The formation constants of some transition metal ions Cr(III), Mn(II), Fe(III), Ni(II) and Cu(II) binary complexes containing Schiff bases resulting from condensation of salicylaldehyde with aniline (I), 2-aminopyridine (II), 4-aminopyridine (III) and 2-aminopyrimidine (IV) were determined pH-metrically in ethanolic medium
Kamaljit Singh et al.
European journal of medicinal chemistry, 52, 82-97 (2012-03-31)
2-Aminopyrimidine based 4-aminoquinolines were synthesized using an efficacious protocol. Some of the compounds showed in vitro anti-plasmodial activity against drug-sensitive CQ(S) (3D7) and drug-resistant CQ(R) (K1) strains of Plasmodium falciparum in the nM range. In particular, 5-isopropyloxycarbonyl-6-methyl-4-(2-nitrophenyl)-2-[(7-chloroquinolin-4-ylamino)butylamino] pyrimidine depicted the lowest
Teodor Silviu Balaban et al.
Journal of the American Chemical Society, 125(14), 4233-4239 (2003-04-03)
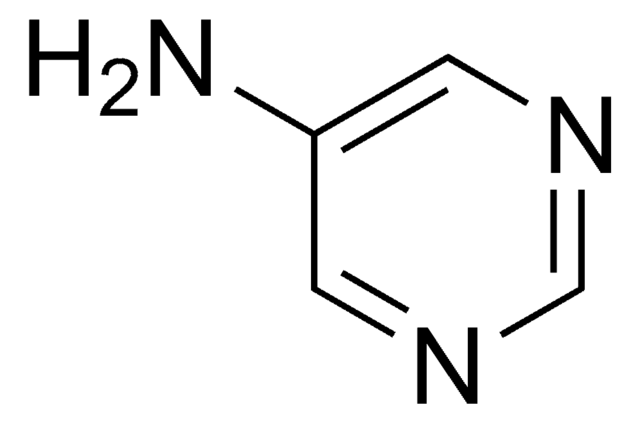
The 2-aminopyrimidin-5-yl ligand is revealed to be a promising candidate for the construction of supramolecular porphyrin arrays with broad absorption bands for efficient light-harvesting. 10-Mono- and 10,20-di(2-aminopyrimidin-5-yl) derivatives of 5,15-bis(3,5-di-tert-butylphenyl)porphyrin have been synthesized in high yield. Their Zn(II) salts show
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service