855286
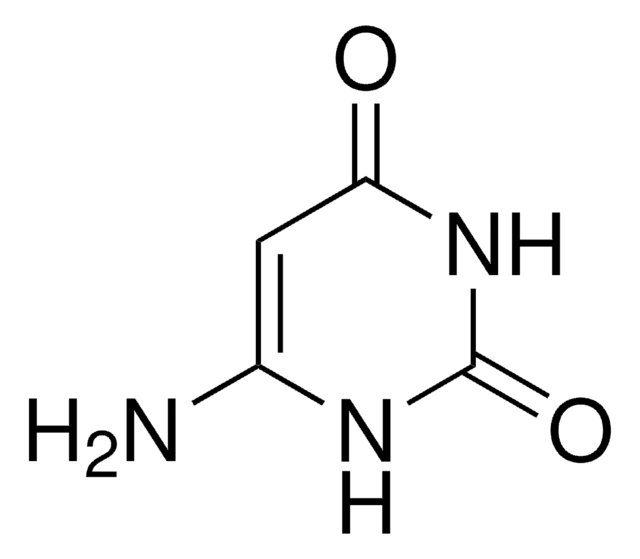
5-Aminouracil
98%
Synonym(s):
5-Amino-2,4-dihydroxypyrimidine, 5-Amino-2,4-pyrimidinediol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
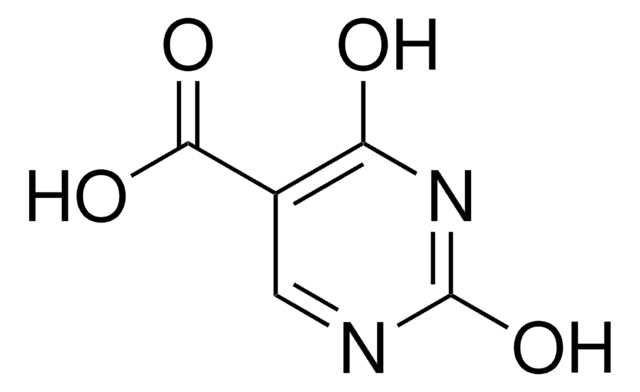
C4H5N3O2
CAS Number:
Molecular Weight:
127.10
Beilstein:
127250
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
powder
mp
>300 °C (lit.)
SMILES string
NC1=CNC(=O)NC1=O
InChI
1S/C4H5N3O2/c5-2-1-6-4(9)7-3(2)8/h1H,5H2,(H2,6,7,8,9)
InChI key
BISHACNKZIBDFM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Paulina Spisz et al.
International journal of molecular sciences, 21(17) (2020-09-05)
Hypoxia-a hallmark of solid tumors-dramatically impairs radiotherapy, one of the most common anticancer modalities. The adverse effect of the low-oxygen state can be eliminated by the concomitant use of a hypoxic cell radiosensitizer. In the present paper, we show that
D Suciu
The International journal of biochemistry, 23(11), 1245-1249 (1991-01-01)
1. The results of this study have contributed to the definition of three categories of chemical inhibitors of DNA replication in mammalian cells. 2. Inhibitors of replicon cluster initiation [4-nitroquinoline-N-oxide (4-NQO), etoposide (VP-16), teniposide (VM-26), amsacrine (m-AMSA), N-methyl-N'-nitro-N-nitrozoguanidine (MNNG), cis-Pt(II)diammine
A González-Fernández et al.
Mutation research, 149(2), 275-281 (1985-04-01)
Proliferating plant cells treated during the late S period with 5-aminouracil (AU), give the typical response that DNA-damaging agents induce, characterized by: an important mitotic delay, and a potentiation of the chromosome damage by caffeine post-treatment. The study of labelled
F Cortés et al.
Experimental cell research, 148(2), 503-507 (1983-10-15)
Meristematic cells of Allium cepa were treated with 5-amino-uracil (5-AU) while incorporating 5-bromodeoxyuridine (BrdU) into DNA until complete inhibition of mitosis was obtained. The pattern of BrdU substitution in interphase nuclei detected by FPG technique in the cells so treated
J E Thomas et al.
Cell and tissue kinetics, 16(3), 285-301 (1983-05-01)
The influence of 5-amino uracil (5-AU) was investigated on the cell cycle of log growth and division-synchronized Tetrahymena pyriformis GL. The division index of log growth phase Tetrahymena was suppressed by 50% after 40 min in 8 mM 5-AU. Cells
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service