747130
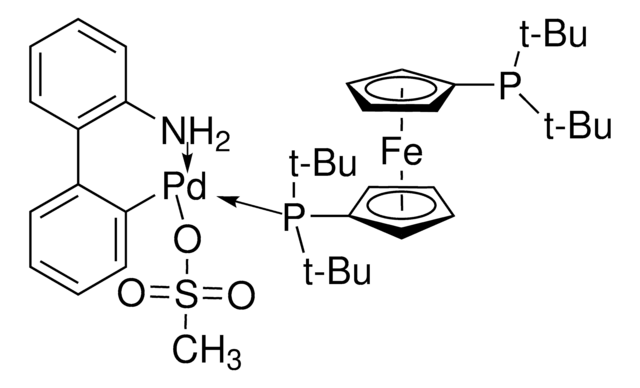
Josiphos SL-J009-1 Pd G3
Synonym(s):
Josiphos SL-J009-1-G3-palladacycle, {(R)-1-[(Sp)-2-(Dicyclohexylphosphino)ferrocenyl]ethyldi-tert-butylphosphine}[2-(2′-amino-1,1′-biphenyl)]palladium(II) methanesulfonate
About This Item
Recommended Products
form
solid
Quality Level
feature
generation 3
reaction suitability
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Cross Couplings
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
mp
180-186 °C
functional group
phosphine
SMILES string
NC1=C(C=CC=C1)C2=C([Pd]OS(C)(=O)=O)C=CC=C2.C[C@@H](P(C(C)(C)C)C(C)(C)C)[C]3[C](P(C4CCCCC4)C5CCCCC5)[C][C][C]3.[C]6[C][C][C][C]6.[Fe]
InChI
1S/C27H47P2.C12H10N.C5H5.CH4O3S.Fe.Pd/c1-21(29(26(2,3)4)27(5,6)7)24-19-14-20-25(24)28(22-15-10-8-11-16-22)23-17-12-9-13-18-23;13-12-9-5-4-8-11(12)10-6-2-1-3-7-10;1-2-4-5-3-1;1-5(2,3)4;;/h14,19-23H,8-13,15-18H2,1-7H3;1-6,8-9H,13H2;1-5H;1H3,(H,2,3,4);;/q;;;;;+1/p-1/t21-;;;;;/m1...../s1
InChI key
GJFJVCUFUWXFEB-RPDSBUJNSA-M
General description
Application
- Total Syntheses of N-Paspaline and N-Emindole PB: Discusses the use of various catalysts including RuPhos and Josiphos ligands for synthesizing complex molecular structures (Radical-Polar Crossover Cyclizations, 2020).
- Unprotected Indazoles Are Resilient to Ring-Opening Isomerization: A Case Study on Catalytic C-S Couplings in the Presence of Strong Base: Utilizes Josiphos SL-J009-1 Pd-G3 for catalytic C-S coupling reactions under strong base conditions (The Journal of Organic Chemistry, 2017).
- Synthesis and characterization of an isopropylBippyPhos precatalyst: Compares various phosphine ligands including XPhos and Josiphos for catalytic activity in organic syntheses (Tetrahedron, 2022).
- Novel quorum sensing inhibitors targeting PqsR: Mentions the experimental use of Josiphos ligands in the synthesis of potential pharmaceutical compounds (2020, University of Saarland).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
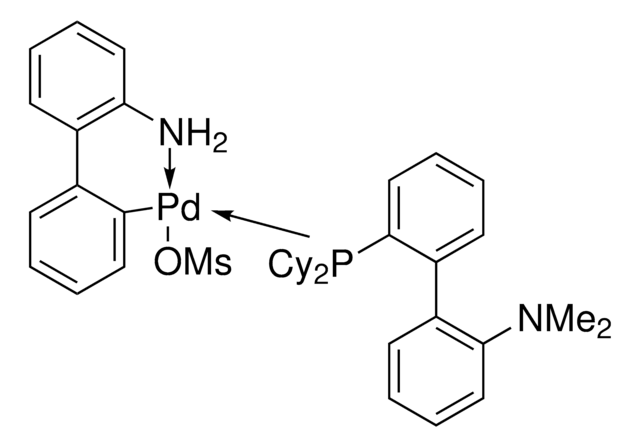
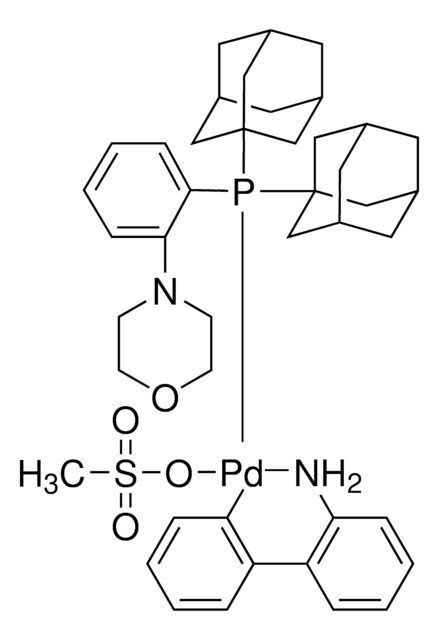
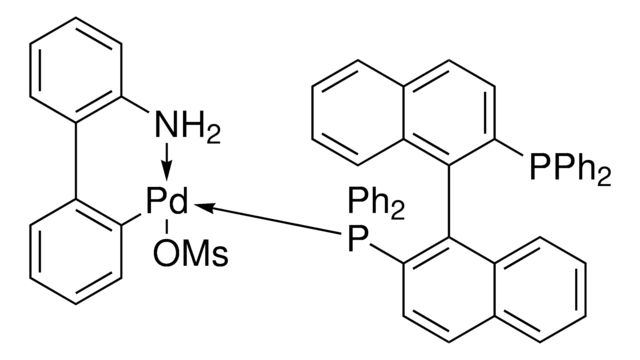
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![(R)-1-[(SP)-2-(Dicyclohexylphosphino)ferrocenyl]ethyldi-tert-butylphosphine ≥97%](/deepweb/assets/sigmaaldrich/product/structures/809/974/e027b628-7c2e-4bde-be7e-f9298d0c8b04/640/e027b628-7c2e-4bde-be7e-f9298d0c8b04.png)
![(R)-1-[(SP)-2-(Diphenylphosphino)ferrocenyl]ethyldicyclohexylphosphine ≥97%](/deepweb/assets/sigmaaldrich/product/structures/245/493/2ae2dd8a-65cc-4aba-9a1f-1292eb1ad8e0/640/2ae2dd8a-65cc-4aba-9a1f-1292eb1ad8e0.png)

![(R)-1-[(SP)-2-(Diphenylphosphino)ferrocenyl]ethyldi-tert-butylphosphine ≥97%](/deepweb/assets/sigmaaldrich/product/structures/168/768/54a48841-6fe6-437a-81af-8c2e54117ef3/640/54a48841-6fe6-437a-81af-8c2e54117ef3.png)


![Mesyl[(tri-t-butylphosphine)-2-(2-aminobiphenyl)]palladium(II)](/deepweb/assets/sigmaaldrich/product/structures/358/298/6539c19e-808c-4cd1-b9e8-19c6928f2384/640/6539c19e-808c-4cd1-b9e8-19c6928f2384.png)

![(R)-1-[(SP)-2-(Dicyclohexylphosphino)ferrocenyl]ethyldicyclohexylphosphine ≥97%](/deepweb/assets/sigmaaldrich/product/structures/156/571/6166b550-075e-4d67-b972-a85cb50b2b22/640/6166b550-075e-4d67-b972-a85cb50b2b22.png)
![[(1,3,5,7-Tetramethyl-6-phenyl-2,4,6-trioxa-6-phosphaadamantane)-2-(2′-amino-1,1′-biphenyl)]palladium(II) methanesulfonate](/deepweb/assets/sigmaaldrich/product/structures/324/001/3ffb4bd2-9c6b-451c-80ee-a217f03ca932/640/3ffb4bd2-9c6b-451c-80ee-a217f03ca932.png)