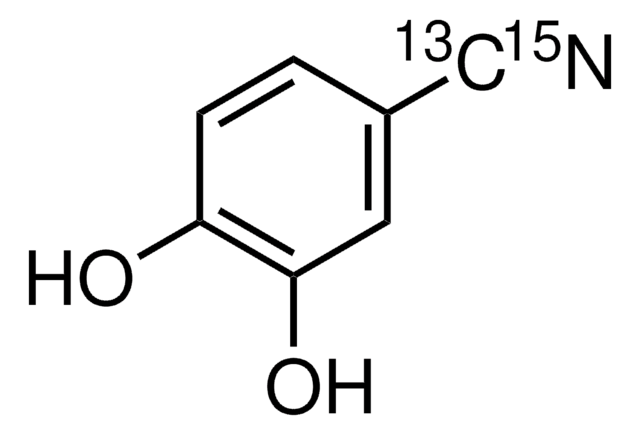
538396
3,4-Dihydroxybenzonitrile
97%
Synonym(s):
Protocatechuonitrile
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(OH)2C6H3CN
CAS Number:
Molecular Weight:
135.12
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
155-159 °C (lit.)
functional group
nitrile
SMILES string
Oc1ccc(cc1O)C#N
InChI
1S/C7H5NO2/c8-4-5-1-2-6(9)7(10)3-5/h1-3,9-10H
InChI key
NUWHYWYSMAPBHK-UHFFFAOYSA-N
General description
3,4-Dihydroxybenzonitrile can be prepared from 4-hydroxy-3-methoxybenzonitrile. It can also be synthesized by reacting 3,4-dimethoxybenzonitrile, lithium diisopropylamide (LDA) and 1,3-dimethyl-2-imidazolidinone (DMEU).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
M J Nelson et al.
Biochemistry, 34(46), 15219-15229 (1995-11-21)
Ferric soybean lipoxygenase forms stable complexes with 4-substituted catechols. The structure of the complex between the enzyme and 3,4-dihydroxybenzonitrile has been studied by resonance Raman, electron paramagnetic resonance, visible, and X-ray spectroscopies. It is a bidentate iron-catecholate complex with at
Sodium Bis (trimethylsilyl) amide and Lithium Diisopropylamide in Deprotection of Alkyl Aryl Ethers: a-Effect of Silicon
Hwu JR, et al.
The Journal of Organic Chemistry, 62.12 , 4097-4104 (1997)
The synthetic technology of 3, 4-dihydroxybenzonitrile
WEI HW, et al.
Fine and Specialty Chemicals / Jing Xi Yu Zhuan Yong Hua Xue Pin, 9, 012-012 (2011)
M M Wick et al.
Journal of pharmaceutical sciences, 76(7), 513-515 (1987-07-01)
This report describes a structure-activity analysis of isomers of three classes of dihydroxybenzene derivatives, including dihydroxybenzaldoxime, dihydroxybenzaldehyde, and dihydroxybenzonitrile. These derivatives were examined for their effect on ribonucleotide reductase activity, macromolecular synthesis, cell growth, and in vivo antitumor activity against
The Reactivity and Reaction Pathway of Fenton Reactions Driven by Substituted 1,2-Dihydroxybenzenes.
Pablo Salgado et al.
Environmental science & technology, 51(7), 3687-3693 (2017-03-09)
Fenton systems are interesting alternatives to advanced oxidation processes (AOPs) applied in soil or water remediation. 1,2-Dihydroxybenzenes (1,2-DHBs) are able to amplify the reactivity of Fenton systems and have been extensively studied in biological systems and for AOP applications. To
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service