480096
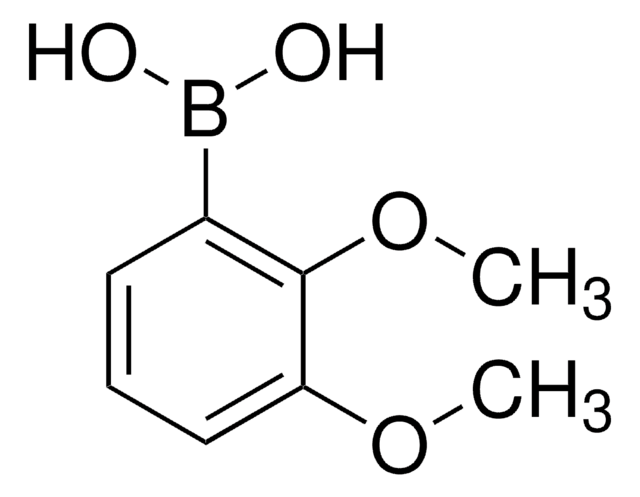
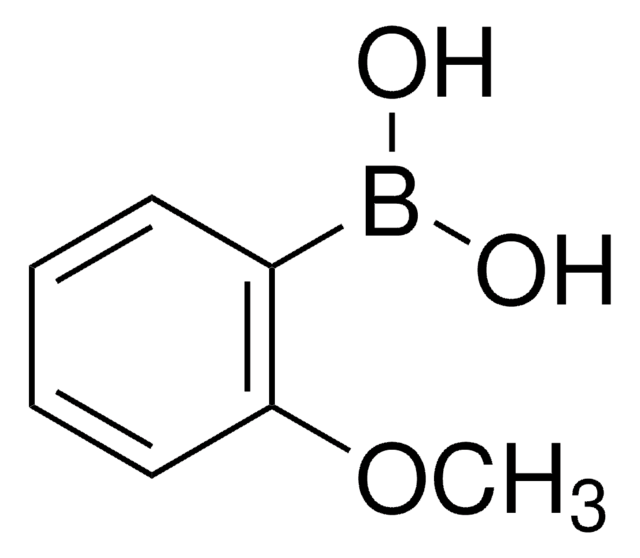
2,6-Dimethoxyphenylboronic acid
≥97%
Synonym(s):
2,6-Dimethoxybenzeneboronic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(CH3O)2C6H3B(OH)2
CAS Number:
Molecular Weight:
181.98
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥97%
mp
110-112 °C (lit.)
SMILES string
COc1cccc(OC)c1B(O)O
InChI
1S/C8H11BO4/c1-12-6-4-3-5-7(13-2)8(6)9(10)11/h3-5,10-11H,1-2H3
InChI key
BKWVXPCYDRURMK-UHFFFAOYSA-N
Application
2,6-Dimethoxyphenylboronic acid can be used as a reagent in:
- The palladium-catalyzed Suzuki-Miyaura coupling reaction to construct carbon-carbon bond.
- The synthesis of 2H-imidazo[1,5-a]pyridin-4-ium bromides, which are utilized as precursors for the preparation of N-heterocyclic carbene ligands.
- The preparation of monosubstituted benzothiazoloquinazolinones as potential monoamine oxidases inhibitors.
Reactant for:
- Suzuki-Miyaura reactions
Other Notes
Contains varying amounts of anhydride
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Palladium catalyzed Suzuki-Miyaura coupling with aryl chlorides using a bulky phenanthryl N-heterocyclic carbene ligand
Song C, et al.
Tetrahedron, 61(31), 7438-7446 (2005)
Imidazo [1, 5-a] pyridine-3-ylidenes-pyridine derived N-heterocyclic carbene ligands
Burstein, C, et al.
Tetrahedron, 61(26), 6207-6217 (2005)
Synthesis of 2-Aryl-12H-benzothiazolo [2, 3-b] quinazolin-12-ones and Their Activity Against Monoamine Oxidases
Jafari B, et al.
ChemistrySelect, 4(37), 11071-11076 (2019)
Ken T Ngo et al.
Journal of the American Chemical Society, 139(7), 2604-2618 (2017-01-25)
Electrocatalytic reduction of CO
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![{[(4-METHYLPHENYL)SULFONYL]OXY}METHYL 4-METHYLBENZENESULFONATE AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/179/050/721713de-e6c4-4f82-9efd-95cbdfd80d69/640/721713de-e6c4-4f82-9efd-95cbdfd80d69.png)