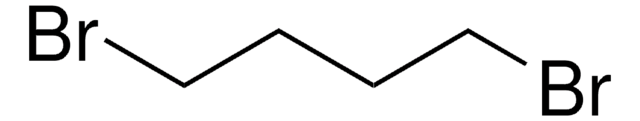
382205
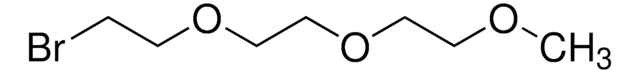
2-Bromoethyl ether
technical grade, 90%
Synonym(s):
Bis(2-bromoethyl) ether
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(BrCH2CH2)2O
CAS Number:
Molecular Weight:
231.91
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Assay
90%
form
liquid
bp
92-93 °C/12 mmHg (lit.)
density
1.845 g/mL at 25 °C (lit.)
functional group
bromo
ether
storage temp.
2-8°C
SMILES string
BrCCOCCBr
InChI
1S/C4H8Br2O/c5-1-3-7-4-2-6/h1-4H2
InChI key
FOZVXADQAHVUSV-UHFFFAOYSA-N
General description
2-Bromoethyl ether (2,2′-Dibromodiethyl ether) is a halogen containing ether. 2,2′-Dibromodiethyl ether has been prepared by reacting dioxane with anhydrous, bromine free hydrogen bromide.
Application
2-Bromoethyl ether (2,2′-Dibromodiethyl ether) may be used in the preparation of 1,4,7-trioxa-10-19-dithia-13,16-diaza-12,17-dioxo-8,9,14,15,20,21-tribenzoheneicosane.
2-Bromoethyl ether may be used in the synthesis of macroheterocycles including redox-active tetrathiafulvalene linked systems and polyoxaza cryptands.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
185.0 °F
Flash Point(C)
85 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
J. Chem. Soc. Perkin Trans. II, 1213-1213 (1989)
Journal of the Chemical Society. Chemical Communications, 1550-1550 (1992)
The reaction of dioxane with hydrogen bromide.
Cleave ABV and Blake RI.
Canadian Journal of Chemistry, 29(9), 785-789 (1951)
Ping-Yu Wu et al.
The Journal of organic chemistry, 71(2), 833-835 (2006-01-18)
[reaction: see text] A mild asymmetric arylation of aromatic aldehydes catalyzed by gamma-amino thiol 5 gave the corresponding diarylmethanols with 95 to >99.5% ee.
New approach to the synthesis of dibenzodithia-and benzothiaazacrown ethers via the aromatization of 2-alkylthio (arylthio) cyclohexanes during bromination.
Kudryatsev KV and Samofin VV.
Chemistry of Heterocyclic Compounds, 33(1), 106-111 (1997)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![Bis[2-(2-chloroethoxy)ethyl] ether ≥99.0% (T)](/deepweb/assets/sigmaaldrich/product/structures/333/320/46ff3398-7a62-42b5-b9bc-0a3d0cb0429c/640/46ff3398-7a62-42b5-b9bc-0a3d0cb0429c.png)