337757
N-(Phosphonomethyl)glycine
96%, for peptide synthesis
Synonym(s):
Glyphosate
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Linear Formula:
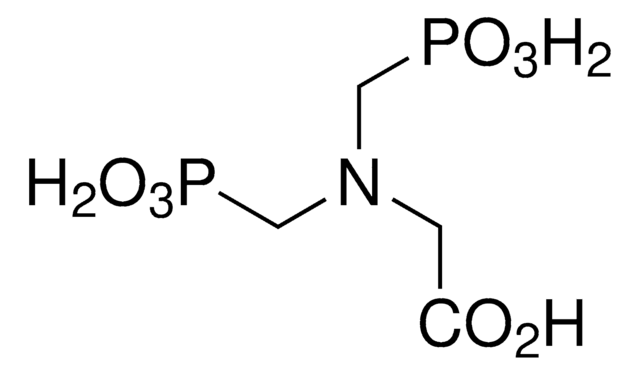
(HO)2P(O)CH2NHCH2CO2H
CAS Number:
Molecular Weight:
169.07
Beilstein:
2045054
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Product Name
N-(Phosphonomethyl)glycine, 96%
Assay
96%
form
powder
reaction suitability
reaction type: solution phase peptide synthesis
mp
230 °C (dec.) (lit.)
application(s)
peptide synthesis
SMILES string
OC(=O)CNCP(O)(O)=O
InChI
1S/C3H8NO5P/c5-3(6)1-4-2-10(7,8)9/h4H,1-2H2,(H,5,6)(H2,7,8,9)
InChI key
XDDAORKBJWWYJS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
N-(Phosphonomethyl)glycine (Glyphosate) is a broad spectrum, non-selective systemic herbicide. It plays an important role in inhibiting the activity of 5-enolpyruvylshikimic acid-3-phosphate synthase, which is involved in aromatic amino acid biosynthesis. Due to its chelation property, glyphosate can form coordinate complexes with a wide range of metal ions.
Application
- Water Pollution Analysis: Campanale et al. assessed glyphosate and AMPA pesticides in river waters and sediments, providing crucial data on the environmental distribution and persistence of N-(Phosphonomethyl)glycine derivatives. This research supports efforts to monitor and regulate environmental pollutants effectively (Campanale et al., 2024).
- Public Health Studies: Urinary biomonitoring of glyphosate exposure was conducted among farmers, utilizing N-(Phosphonomethyl)glycine as a marker. This study contributes to our understanding of occupational exposure risks and supports the development of health safety guidelines (Chang et al., 2024).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Aquatic Chronic 2 - Eye Dam. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Hui Gao et al.
Journal of applied toxicology : JAT, 39(8), 1096-1107 (2019-03-26)
Glyphosate-based herbicides have been used worldwide for decades and have been suggested to induce nephrotoxicity, but the underlying mechanism is not yet clear. In this study, we treated a human renal proximal tubule cell line (HK-2) with glyphosate for 24 hours
The herbicide glyphosate is a potent inhibitor of 5-enolpyruvylshikimic acid-3-phosphate synthase.
Steinrucken H C and Amrhein N
Biochemical and Biophysical Research Communications, 94(4), 1207-1212 (1980)
Copper (II) complexes of N-(phosphonomethyl) glycine in aqueous solution: a thermodynamic and spectrophotometric study.
Daniele P G, et al.
Talanta, 45(2), 425-431 (1997)
Biochemical effects of glyphosate (N-(phosphonomethyl) glycine)[Lemna, higher plants, phenylalanine ammonialyase].
Hoagland R E and Duke S O
ACS Symp. Ser. (1982)
The complex chemistry of N-(phosphonomethyl) glycine (glyphosate): preparation and characterization of the ammonium, lithium, sodium (4 polymorphs) and silver (I) complexes.
Sagatys D S, et al.
J. Chem. Soc., Dalton Trans., 19, 3404-3410 (2000)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service