All Photos(1)
About This Item
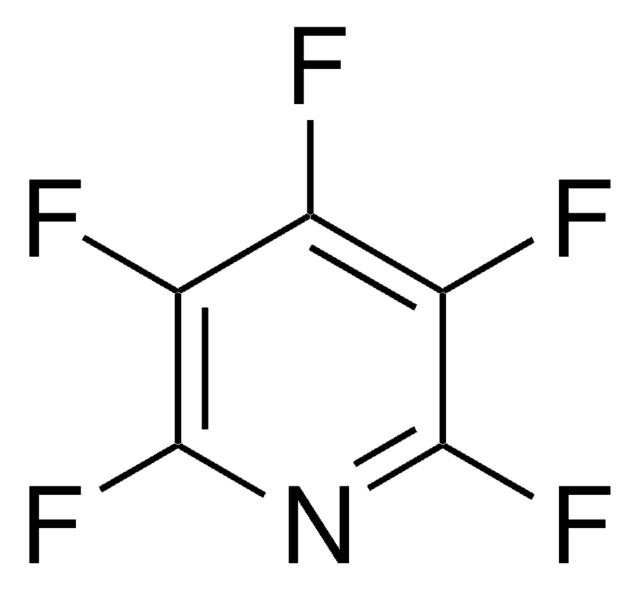
Empirical Formula (Hill Notation):
C6H3F3
CAS Number:
Molecular Weight:
132.08
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98%
form
liquid
refractive index
n20/D 1.423 (lit.)
bp
94-95 °C (lit.)
density
1.28 g/mL at 25 °C (lit.)
SMILES string
Fc1cccc(F)c1F
InChI
1S/C6H3F3/c7-4-2-1-3-5(8)6(4)9/h1-3H
InChI key
AJKNNUJQFALRIK-UHFFFAOYSA-N
General description
Crystal structure 1,2,3-trifluorobenzene was reported. The microwave spectrum of 1,2,3-trifluorobenzene was studied using a molecular beam Fourier transform microwave spectrometer. Laser-induced fluorescence spectra of the radical cation of 1,2,3-trifluorobenzene was determined in both the gas phase and solid Ne matrices.
accessory
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
24.8 °F - closed cup
Flash Point(C)
-4 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Michael T Kirchner et al.
Acta crystallographica. Section E, Structure reports online, 65(Pt 11), o2670-o2670 (2009-01-01)
In the title compound, C(6)H(3)F(3), weak electrostatic and dispersive forces between C(δ+)-F(δ-) and H(δ+)-C(δ-) groups are at the borderline of the hydrogen-bond phenomenon and are poorly directional and further deformed in the presence of π-π stacking inter-actions. The mol-ecule lies
Microwave Spectrum of 1, 2, 3-Trifluorobenzene.
Onda M, et al.
Journal of Molecular Spectroscopy, 169(2), 480-483 (1995)
Marissa A Dobulis et al.
The Journal of chemical physics, 152(20), 204309-204309 (2020-06-04)
The broadband photoelectron source realized by detaching O2-·X (X = neutral unsaturated molecule) complexes offers a unique opportunity to probe temporary anion states of the unsaturated species. Detachment of the ion molecule complex typically accesses a dissociative portion of the
The laser-induced fluorescence spectrum of the 1, 2, 3-trifluorobenzene radical cation.
Bondybey VE, et al.
Journal of Molecular Spectroscopy, 84(1), 124-131 (1980)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service