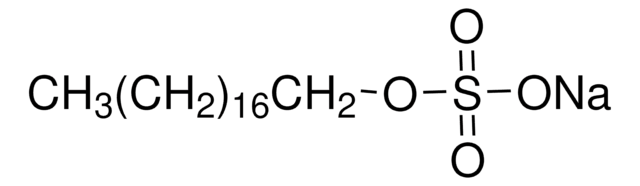318183
Methyl sulfate sodium salt
Synonym(s):
Monomethyl ester sulfuric acid sodium salt, Sodium methyl sulfate
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
CH3OSO3Na
CAS Number:
Molecular Weight:
134.09
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
powder
Quality Level
impurities
<3% methanol
<5% water
mp
210 °C (lit.)
SMILES string
[Na+].COS([O-])(=O)=O
InChI
1S/CH4O4S.Na/c1-5-6(2,3)4;/h1H3,(H,2,3,4);/q;+1/p-1
InChI key
DZXBHDRHRFLQCJ-UHFFFAOYSA-M
Application
Methyl sulfate sodium salt can be used to synthesize:
- Methylsulfate anion based 1,3,4-trialkyl-1,2,3-triazolium ionic liquids for use in Morita–Baylis–Hillman reaction.
- Anisole by reacting with phenol.
- Hydroxyanilino quinolines for use as RET kinase inhibitors.
Reactant or reagent involved in:
- Studying lamellar structure formation in hybrid nanomaterials created by miniemulsion
- EPR studies of radical ions radiolytically generated from ionic liquids, used as a reference
- Micellular studies specifically the rate-retarding effects on hydrolysis of substituted 1-benzoyl-1,2,4-triazoles and self-assembly / microstructure of mixed micelles
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of new triazolium-based ionic liquids and their use in the Morita-Baylis-Hillman reaction
F Alioune, et al.
Tetrahedron Letters, 56(36), 5128-5131 (2015)
Synthesis of anisole from phenol and sodium methyl sulfate, a byproduct from synthesis of medicine intermediates
Guoping W, et al.
Petrochemical Technology, 45(11), 1337-1337 (2016)
The discovery of substituted 4-(3-hydroxyanilino)-quinolines as potent RET kinase inhibitors
Robinett R G, et al.
Bioorganic & Medicinal Chemistry Letters, 17(21), 5886-5893 (2007)
Jesús Oria-Hernández et al.
The Journal of biological chemistry, 280(45), 37924-37929 (2005-09-09)
For more than 50 years, it has been known that K(+) is an essential activator of pyruvate kinase (Kachmar, J. F., and Boyer, P. D. (1953) J. Biol. Chem. 200, 669-683). However, the role of K(+) in the catalysis by
Emiliana Damian Risberg et al.
Dalton transactions (Cambridge, England : 2003), (8)(8), 1328-1338 (2009-05-26)
The strongly hydrogen bonded species (CH3)2SO...H3O+ formed in concentrated hydrochloric acid displays a new low energy feature in its sulfur K-edge X-ray absorption near edge structure (XANES) spectrum. Density Functional Theory-Transition Potential (DFT-TP) calculations reveal that the strong hydrogen bonding
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service