SRP3032
Enterokinase human
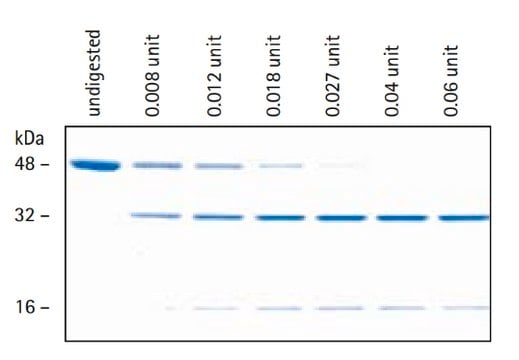
recombinant, expressed in CHO cells, ≥90% (SDS-PAGE), ≥90% (HPLC), suitable for cell culture
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Código UNSPSC:
12352204
NACRES:
NA.32
Productos recomendados
origen biológico
human
recombinante
expressed in CHO cells
Ensayo
≥90% (HPLC)
≥90% (SDS-PAGE)
Formulario
lyophilized
mol peso
108.7 kDa
envase
pkg of 50 μg
técnicas
cell culture | mammalian: suitable
impurezas
<0.1 EU/μg endotoxin, tested
color
white to off-white
Nº de acceso UniProt
Condiciones de envío
wet ice
temp. de almacenamiento
−20°C
Información sobre el gen
human ... TMPRSS15(5651)
Descripción general
Human Enterokinase is expressed as a linear 1019 amino acid polypeptide precursor glycoprotein. Proteolytic processing of this precursor generates the biologically active form of enterokinase, which consists of two polypeptide chains (heavy chain and light chain) held together by a single disulfide bond, resulting in formation of a biologically active heterodimer. The heavy chain consists of 784 amino acid residues, and the light consists of 235 amino acid residues. The mRNA is present in the proximal small intestine, and the protein is in enterocytes of the duodenum and proximal jejunum. The gene is mapped to human chromosome 21q21.
Acciones bioquímicas o fisiológicas
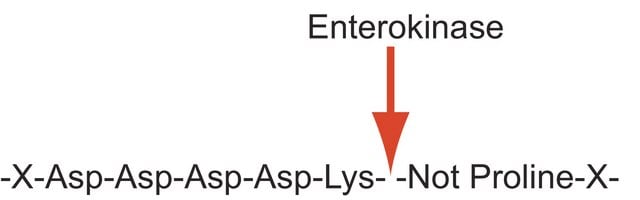
Proteases (also called proteolytic enzymes, peptidases, or proteinases) are enzymes that hydrolyze the amide bonds within proteins or peptides. Most proteases act in a specific manner, hydrolyzing bonds at or adjacent to specific residues or a specific sequence of residues contained within the substrate protein or peptide. Proteases play an important role in most diseases and biological processes including prenatal and postnatal development, reproduction, signal transduction, the immune response, various autoimmune and degenerative diseases, and cancer. Enterokinase sequentially cleaves carboxyl side of D-D-D-D-K. It is a serine protease which mainly activates trypsinogen.
Secuencia
Heavy chain: LTIKESQRGA ALGQSHEARA TFKITSGVTY NPNLQDKLSV DFKVLAFDLQ QMIDEIFLSS NLKNEYKNSR VLQFENGSII VVFDLFFAQW VSDQNVKEEL IQGLEANKSS QLVTFHIDLN SVDILDKLTT TSHLATPGNV SIECLPGSSP CTDALTCIKA DLFCDGEVNC PDGSDEDNKM CATVCDGRFL LTGSSGSFQA THYPKPSETS VVCQWIIRVN QGLSIKLSFD DFNTYYTDIL DIYEGVGSSK ILRASIWETN PGTIRIFSNQ VTATFLIESD ESDYVGFNAT YTAFNSSELN NYEKINCNFE DGFCFWVQDL NDDNEWERIQ GSTFSPFTGP NFDHTFGNAS GFYISTPTGP GGRQERVGLL SLPLDPTLEP ACLSFWYHMY GENVHKLSIN ISNDQNMEKT VFQKEGNYGD NWNYGQVTLN ETVKFKVAFN AFKNKILSDI ALDDISLTYG ICNGSLYPEP TLVPTPPPEL PTDCGGPFEL WEPNTTFSST NFPNSYPNLA FCVWILNAQK GKNIQLHFQE FDLENINDVV EIRDGEEADS LLLAVYTGPG PVKDVFSTTN RMTVLLITND VLARGGFKAN FTTGYHLGIP EPCKADHFQC KNGECVPLVN LCDGHLHCED GSDEADCVRF FNGTTNNNGL VRFRIQSIWH TACAENWTTQ ISNDVCQLLG LGSGNSSKPI FSTDGGPFVK LNTAPDGHLI LTPSQQCLQD SLIRLQCNHK SCGKKLAAQD ITPK Light Chain: IVGGSNAKEG AWPWVVGLYY GGRLLCGASL VSSDWLVSAA HCVYGRNLEP SKWTAILGLH MKSNLTSPQT VPRLIDEIVI NPHYNRRRKD NDIAMMHLEF KVNYTDYIQP ICLPEENQVF PPGRNCSIAG WGTVVYQGTT ANILQEADVP LLSNERCQQQ MPEYNITENM ICAGYEEGGI DSCQGDSGGP LMCQENNRWF LAGVTSFGYK CALPNRPGVY ARVSRFTEWI QSFLH
Forma física
Lyophilized from 10 mM Sodium Phosphate, pH 7.5 + 1 mM Calcium Chloride.
Reconstitución
Centrifuge the vial prior to opening. Reconstitute in water to a concentration of 0.1-1.0 mg/ml. Do not vortex. This solution can be stored at 2-8°C for up to 1 week. For extended storage, it is recommended to store in working aliquots at -20°C to -80°C.
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
Lot/Batch Number
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Biochemical characterization of human enteropeptidase light chain.
Gasparian ME, et al.
Biochemistry (Moscow), 71, 113-119 (2006)
Production and purification of recombinant enteropeptidase expressed in an insect-baculovirus cell system.
Azhar M and Somashekhar R
Preparative Biochemistry & Biotechnology, 45, 268-278 (2015)
N S Mann et al.
Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine (New York, N.Y.), 206(2), 114-118 (1994-06-01)
Enterokinase is a glycoprotein and is now designated enteropeptidase (E.C.3.4.4.8.). It is present in the duodenal and jejunal mucosa. Pancreatic proteolytic enzymes are secreted as proenzymes. Enterokinase converts trypsinogen to trypsin in the duodenal lumen. Duodenopancreatic reflux of duodenal enterokinase
Shenoy Rajesh Tulsidas et al.
Acta crystallographica. Section F, Structural biology and crystallization communications, 65(Pt 5), 533-535 (2009-05-02)
Serine proteases play a major role in host-pathogen interactions. The innate immune system is known to respond to invading pathogens in a nonspecific manner. The serine protease cascade is an essential component of the innate immune system of the horseshoe
Valerio Rasi et al.
Frontiers in immunology, 13, 830290-830290 (2022-03-19)
Cytotoxic lymphocytes release proteins contained within the cytoplasmic cytolytic granules after recognition of infected or tumor target cells. These cytotoxic granular proteins (namely granzymes, granulysin, and perforin) are key immunological mediators within human cellular immunity. The availability of highly purified
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico