01673
Acriflavine
suitable for fluorescence, BioReagent, ≥90% (AT)
Sinónimos:
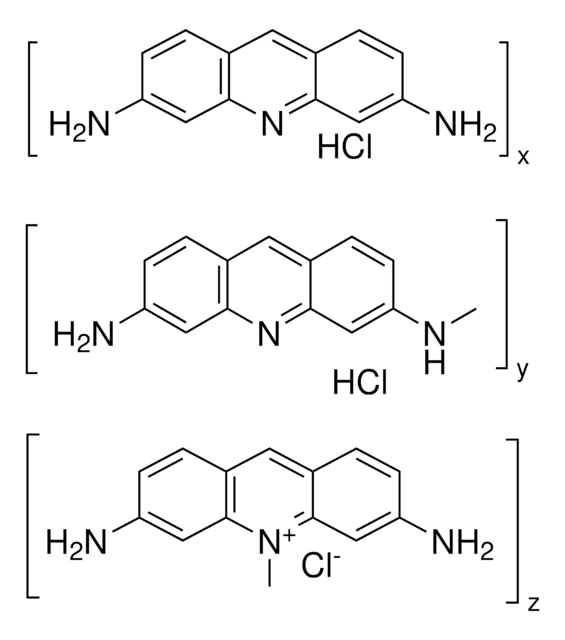
3,6-Diamino-10-methylacridinium chloride mixt. with 3,6-diaminoacridine (proflavine), Euflavine, Trypaflavine Neutral
About This Item
Productos recomendados
product line
BioReagent
assay
≥90% (AT)
form
powder
composition
Sum of components 3,6-diaminoacridinium chloride and 3,6-diamino-10-methylacridinium chloride, ≥70% HPLC
solubility
H2O: 0.33 g/mL (lit.)(lit.)
fluorescence
λex 463 nm; λem 490 nm in ethanol
suitability
suitable for fluorescence
SMILES string
[Cl-].Nc1ccc2cc3ccc(N)cc3nc2c1.C[n+]4c5cc(N)ccc5cc6ccc(N)cc46
InChI
1S/C14H13N3.C13H11N3.ClH/c1-17-13-7-11(15)4-2-9(13)6-10-3-5-12(16)8-14(10)17;14-10-3-1-8-5-9-2-4-11(15)7-13(9)16-12(8)6-10;/h2-8H,1H3,(H3,15,16);1-7H,14-15H2;1H
InChI key
PEJLNXHANOHNSU-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
Excitation and emission wavelengths in various solvents :
- Methanol: λex = 424 nm; λem = 518 nm
- Ethanol: λex = 426 nm; λem = 524 nm
- Propanol: λex = 430 nm; λem = 512 nm
- Butanol: λex = 430 nm; λem = 526 nm
- Formamide: λex = 434 nm; λem = 524 nm
- Glycerol: λex = 432 nm; λem = 540 nm
- Water: λex = 416 nm; λem = 514 nm
Insoluble in ether, chloroform, and fixed oils. Utilized in fluorescence steady state measurements as a donor molecule (when paired with rhodamine 6G as the acceptor) to function as a pH sensor .
Other Notes
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico