37878
Imazaquin
PESTANAL®, analytical standard
Sinónimos:
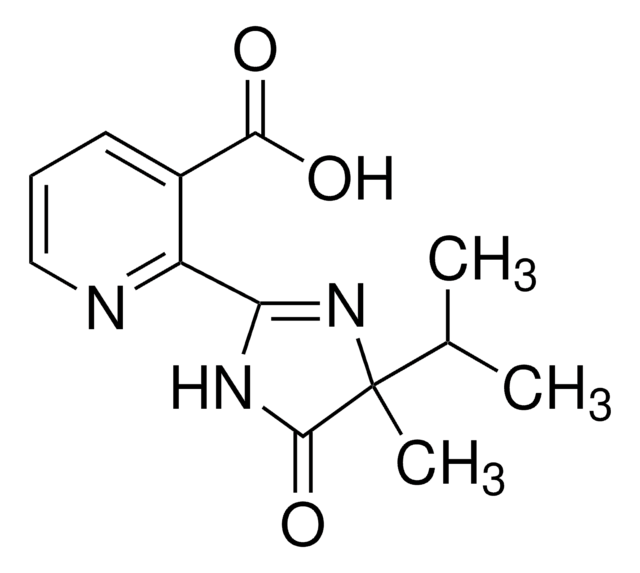
2-(4-Isopropyl-4-methyl-5-oxo-4,5-dihydro-1H-imidazol-2-yl)-quinoline-3-carboxylic acid
About This Item
Productos recomendados
grado
analytical standard
Nivel de calidad
Línea del producto
PESTANAL®
caducidad
limited shelf life, expiry date on the label
técnicas
HPLC: suitable
gas chromatography (GC): suitable
aplicaciones
agriculture
environmental
Formato
neat
cadena SMILES
CC(C)C1(C)N=C(NC1=O)c2nc3ccccc3cc2C(O)=O
InChI
1S/C17H17N3O3/c1-9(2)17(3)16(23)19-14(20-17)13-11(15(21)22)8-10-6-4-5-7-12(10)18-13/h4-9H,1-3H3,(H,21,22)(H,19,20,23)
Clave InChI
CABMTIJINOIHOD-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Aplicación
Productos recomendados
Información legal
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 4 Dermal
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 2
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico