09658
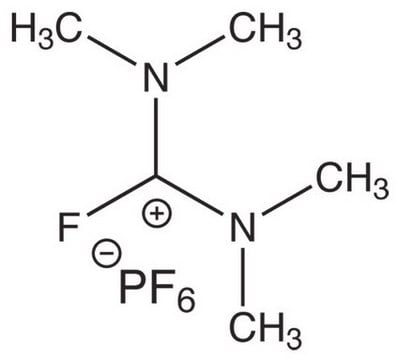
Chloro-N,N,N′,N′-tetramethylformamidinium hexafluorophosphate
≥98.0% (T)
Sinónimos:
TCFH
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C5H12ClF6N2P
Número de CAS:
Peso molecular:
280.58
Beilstein/REAXYS Number:
7896715
MDL number:
UNSPSC Code:
12352101
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
≥98.0% (T)
reaction suitability
reaction type: Coupling Reactions
mp
99-118 °C
application(s)
peptide synthesis
storage temp.
2-8°C
SMILES string
F[P-](F)(F)(F)(F)F.CN(C)\C(Cl)=[N+](\C)C
InChI
1S/C5H12ClN2.F6P/c1-7(2)5(6)8(3)4;1-7(2,3,4,5)6/h1-4H3;/q+1;-1
InChI key
CUKNPSDEURGZCO-UHFFFAOYSA-N
Categorías relacionadas
Application
Chloro-N,N,N′,N′-tetramethylformamidinium hexafluorophosphate can be used as a reactant for the synthesis of:
It can also be used as a reagent for the synthesis of:
- Onium salts for use in peptide coupling.
- Benzotriazole based uranium reagent, a safer replacement for coupling reagents.
It can also be used as a reagent for the synthesis of:
- Cancer cell cytotoxins.
- Bioconjugation reagents.
Other Notes
Coupling reagent for peptide synthesis and starting material for preparing other coupling reagents
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
A novel family of onium salts based upon isonitroso meldrum's acid proves useful as peptide coupling reagents
El-Faham A, et al.
European Journal of Organic Chemistry, 2010(19), 3641-3649 (2010)
An Efficient Second-Generation Manufacturing Process for the pan-RAF Inhibitor Belvarafenib
Zell, D., et al.
Organic Process Research & Development, 25, 10, 2338-2350 (2021)
COMU: a safer and more effective replacement for benzotriazole-based uronium coupling reagents
El-Faham A, et al.
Chemistry?A European Journal , 15(37), 9404-9416 (2009)
Rapid Development of a Commercial Process for Linrodostat, an Indoleamine 2,3-Dioxygenase (IDO) Inhibitor
Fraunhoffer, K.J., et al.
Organic Process Research & Development, 23, 11, 2482-2498 (2019)
Development of a Scalable, Stereoselective Second-Generation Route for CXCR7 Antagonist ACT-1004-1239 via Chiral Enamine Reduction and a Novel Telescoped Sequence of Transesterification, cis-to-trans Epimerization, and Saponification
Schafer, Gabriel et. al.
Organic Process Research & Development, 28(6), 2103?2116-2103?2116 (2024)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico