930067
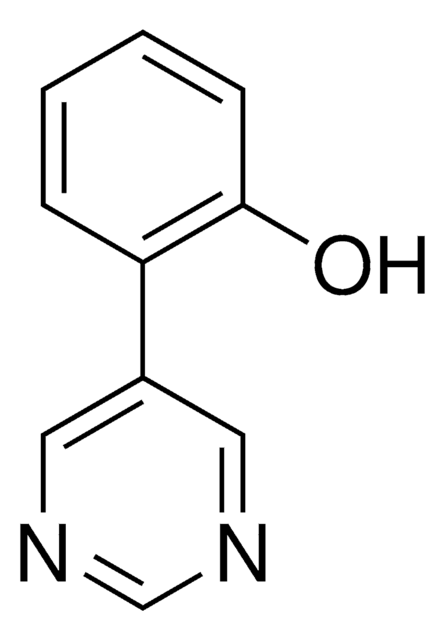
2-(Pyrimidin-5-yl)benzaldehyde
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C11H9N2O
Peso molecular:
185.20
MDL number:
UNSPSC Code:
12352101
NACRES:
NA.06
Productos recomendados
Application
2-(Pyrimidin-5-yl)benzaldehyde is a temporary directing group (TDG) to assist as a co-catalyst for metal catalyzed C-H functionalization. Often in C-H functionalization, an auxiliary compound is used to control site selectivity. These traditionally are covalently bonded to the compound of interest, and must subsequently be removed after functionalization, like a typical protecting group. To simplify the process of C-H functionalization, 2-fluoro-6-(pyrimidin-5-yl)aniline is one of a series of temporary directing groups developed by Deb Maiti′s lab that promote site selectivity without the inclusion of additional synthetic steps.
2-(pyrimidin-5-yl)benzaldehyde is an effective TDG for meta directed C-H functionalization of amine substituted target compounds, with high selectivity.
2-(pyrimidin-5-yl)benzaldehyde is an effective TDG for meta directed C-H functionalization of amine substituted target compounds, with high selectivity.
Other Notes
Imine as a linchpin approach for meta-C–H functionalization
https://www.nature.com/articles/s41570-021-00311-3">Transient directing ligands for selective metal-catalysed C–H activation
https://www.nature.com/articles/s41570-021-00311-3">Transient directing ligands for selective metal-catalysed C–H activation
Related product
Referencia del producto
Descripción
Precios
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Transient directing ligands for selective metal-catalysed C?H activation
Nupur, et al.
Nature Reviews Chemistry, 5, 646?659-646?659 (2021)
Sukdev Bag et al.
Nature communications, 12(1), 1393-1393 (2021-03-04)
Despite the widespread applications of C-H functionalization, controlling site selectivity remains a significant challenge. Covalently attached directing groups (DGs) served as ancillary ligands to ensure ortho-, meta- and para-C-H functionalization over the last two decades. These covalently linked DGs necessitate
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico





![(R)-1-[(SP)-2-(Dicyclohexylphosphino)ferrocenyl]ethyldi-tert-butylphosphine ≥97%](/deepweb/assets/sigmaaldrich/product/structures/809/974/e027b628-7c2e-4bde-be7e-f9298d0c8b04/640/e027b628-7c2e-4bde-be7e-f9298d0c8b04.png)
