901233
(2R)-2-Phenyl-3,4-dihydro-2H-pyrimido[2,1-b][1,3]benzothiazole
≥95%
Sinónimos:
(R)-2-Phenyl-3,4-dihydro-2H-benzo[4,5]thiazolo[3,2-a]pyrimidine, (R)-Homobenzotetramisole, Birman (R)-HBTM
About This Item
Productos recomendados
Nivel de calidad
Ensayo
≥95%
Formulario
powder or chunks
mp
145-147 °C
grupo funcional
phenyl
thioether
temp. de almacenamiento
2-8°C
cadena SMILES
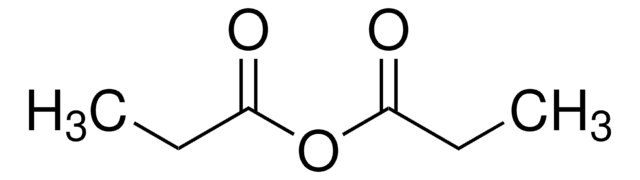
C12=CC=CC=C1SC3=N[C@@H](C4=CC=CC=C4)CCN23
InChI
1S/C16H14N2S/c1-2-6-12(7-3-1)13-10-11-18-14-8-4-5-9-15(14)19-16(18)17-13/h1-9,13H,10-11H2/t13-/m1/s1
Clave InChI
ZMYZJAQMQBHNLH-CYBMUJFWSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Aplicación
Otras notas
Technology Spotlight: Homobenzotetramisole (HBTM): A General Organocatalyst for Asymmetric Acylations
[1] Determination of the Absolute Configuration β-Chiral Primary Alcohols Using the Competing Enantioselective Conversion Method
[2] Asymmetric Catalytic Synthesis of Thiochromenes via an Acyl Transfer-Initiated Cascade
[3] Rapid assembly of complex cyclopentanes employing chiral, α, β-unsaturated acylammonium intermediates
[4] Determination of Absolute Configuration Using Kinetic Resolution Catalysts
[5] Kinetic Resolution of Secondary Alcohols Using Amidine-Based Catalysts
Producto relacionado
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Artículos
We are proud to offer the isothiourea organocatalyst homobenzotetramisole (HBTM) as part of our asymmetric catalysis portfolio in both (R) and (S) enantiomeric forms.
We are proud to offer the isothiourea organocatalyst homobenzotetramisole (HBTM) as part of our asymmetric catalysis portfolio in both (R) and (S) enantiomeric forms.
We are proud to offer the isothiourea organocatalyst homobenzotetramisole (HBTM) as part of our asymmetric catalysis portfolio in both (R) and (S) enantiomeric forms.
We are proud to offer the isothiourea organocatalyst homobenzotetramisole (HBTM) as part of our asymmetric catalysis portfolio in both (R) and (S) enantiomeric forms.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico
![(2S)-2-Phenyl-2,3-dihydroimidazo[2,1-b][1,3]benzothiazole](/deepweb/assets/sigmaaldrich/product/structures/273/874/ec914181-6100-4322-aeb1-7665aaf7518c/640/ec914181-6100-4322-aeb1-7665aaf7518c.png)
![(2S)-2-Phenyl-3,4-dihydro-2H-pyrimido[2,1-b][1,3]benzothiazole 95%](/deepweb/assets/sigmaaldrich/product/structures/403/288/5e981722-19a3-4ad1-b2af-26137b4f5c18/640/5e981722-19a3-4ad1-b2af-26137b4f5c18.png)