901116
trans-Bis(dicyclohexylphenylphosphine)(2-methylphenyl)nickel(II) chloride
Sinónimos:
trans-(PCy2Ph)2Ni(o-tolyl)Cl
About This Item
Productos recomendados
form
powder or solid
reaction suitability
core: nickel
reaction type: Cross Couplings
reagent type: catalyst
mp
174-179 °C
SMILES string
Cl[Ni](P(C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C1([H])[H])(C2=C([H])C([H])=C([H])C([H])=C2[H])C3([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C3([H])[H])(P(C4([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C4([H])[H])(C5=C([H])C([H])=C([H
Other Notes
Legal Information
related product
Storage Class
13 - Non Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
The Jamison group has developed a library of bench-stable phosphine-containing nickel(II) precatalysts that are converted into active catalysts in situ.
Nickel complexes catalyze various synthetic reactions like oxidative addition, C-H activation, and cross-coupling.
Nickel complexes catalyze various synthetic reactions like oxidative addition, C-H activation, and cross-coupling.
Nickel complexes catalyze various synthetic reactions like oxidative addition, C-H activation, and cross-coupling.
Contenido relacionado
Research in the Jamison group is centered on the development of new reactions and technologies for organic synthesis. Towards these themes, the group has pioneered a number of air-stable nickel precatalysts supported by phosphines and N-heterocyclic carbenes that are readily converted to the active catalyst in situ.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico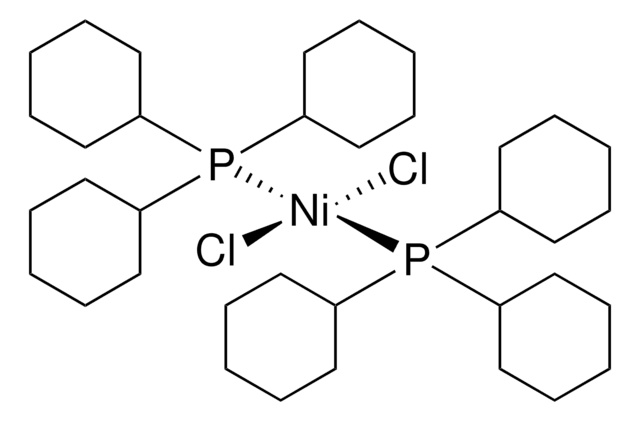

nickel(II) chloride](/deepweb/assets/sigmaaldrich/product/structures/252/197/3c560697-beb3-4c52-85d6-ebc3af13db69/640/3c560697-beb3-4c52-85d6-ebc3af13db69.png)
![[(TMEDA)Ni(o-tolyl)Cl] 95%](/deepweb/assets/sigmaaldrich/product/structures/236/439/768c916e-994f-47e3-a980-3ca0471317d7/640/768c916e-994f-47e3-a980-3ca0471317d7.png)
![[1,2-Bis(diphenylphosphino)ethane]dichloronickel(II)](/deepweb/assets/sigmaaldrich/product/structures/707/956/483e7a6e-5fb5-4e39-abd1-ecf33ccab3cf/640/483e7a6e-5fb5-4e39-abd1-ecf33ccab3cf.png)
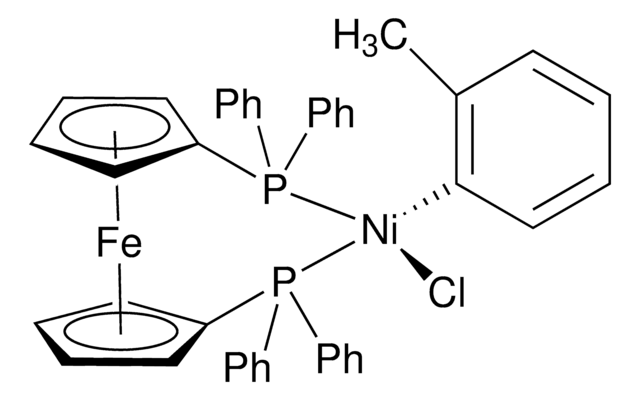
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloronickel(II) 97%](/deepweb/assets/sigmaaldrich/product/structures/274/566/a60d6584-163a-4c41-a738-60f8e4d524fa/640/a60d6584-163a-4c41-a738-60f8e4d524fa.png)