767840
Zinc difluoromethanesulfinate
95%
Sinónimos:
Bis(((difluoromethyl)sulfinyl)oxy)zinc, 1,1-difluoro-methanesulfinic acid zinc salt (2:1), Baran difluoromethylation reagent, DFMS
About This Item
Productos recomendados
Nivel de calidad
Ensayo
95%
Formulario
solid
idoneidad de la reacción
reaction type: C-C Bond Formation
reaction type: Fluorinations
reagent type: catalyst
reaction type: C-H Activation
reagent type: diversification reagent
grupo funcional
fluoro
sulfinic acid
temp. de almacenamiento
2-8°C
cadena SMILES
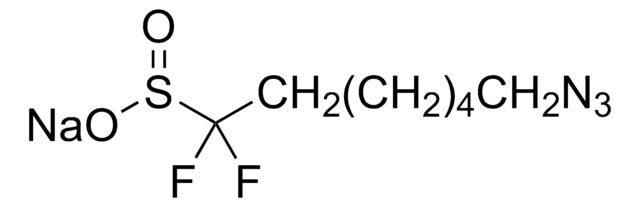
FC(F)S(=O)O[Zn]OS(=O)C(F)F
InChI
1S/2CH2F2O2S.Zn/c2*2-1(3)6(4)5;/h2*1H,(H,4,5);/q;;+2/p-2
Clave InChI
UGEYAPVLXKEKMP-UHFFFAOYSA-L
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Aplicación
Practical and Innate Carbon-Hydrogen Functionalization of Heterocycles
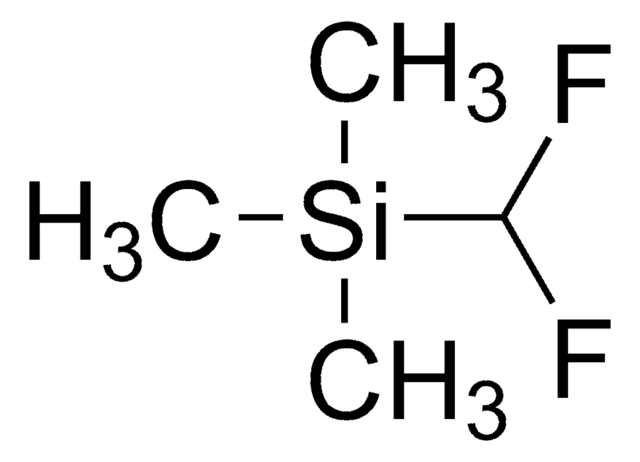
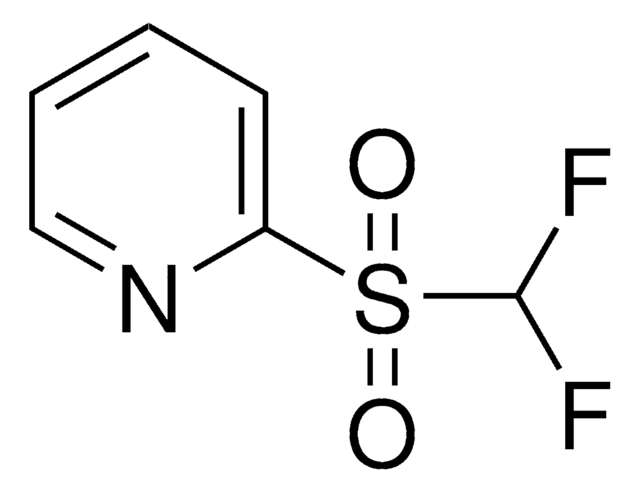
DFMS is a new reagent for direct difluoromethylation of organic substrates via a radical process. This mild, operationally simple, chemoselective, and scalable difluoromethylation method is compatible with a range of nitrogen-containing heteroarene substrates of varying complexity as well as select classes of conjugated p−systems and thiols.†
A New Reagent for Direct Difluoromethylation
Learn More at the Professor and Product Portal of Professor Phil S. Baran.
Ligadura / enlace
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órganos de actuación
Respiratory system
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Rapidly diversify (hetero)aromatic scaffolds for chemical industry needs amid resource and time constraints, ensuring efficiency.
Rapidly diversify (hetero)aromatic scaffolds for chemical industry needs amid resource and time constraints, ensuring efficiency.
Rapidly diversify (hetero)aromatic scaffolds for chemical industry needs amid resource and time constraints, ensuring efficiency.
Rapidly diversify (hetero)aromatic scaffolds for chemical industry needs amid resource and time constraints, ensuring efficiency.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico




![Zinc di[bis(trifluoromethylsulfonyl)imide] 95%](/deepweb/assets/sigmaaldrich/product/structures/336/073/952daadd-0a7c-4bec-bbaf-442a24c62161/640/952daadd-0a7c-4bec-bbaf-442a24c62161.png)