636010
3,5-Dimethylpyrazole-4-boronic acid pinacol ester
97%
Sinónimos:
3,5-Dimethyl-4-(4,4,5,5-tetramethyl-1,3,2dioxaborolan-2-yl)-1H-pyrazole, 3,5-Dimethyl-4-pyrazoleboronic acid pinacol ester
About This Item
Productos recomendados
assay
97%
form
solid
mp
163-168 °C (lit.)
SMILES string
Cc1n[nH]c(C)c1B2OC(C)(C)C(C)(C)O2
InChI
1S/C11H19BN2O2/c1-7-9(8(2)14-13-7)12-15-10(3,4)11(5,6)16-12/h1-6H3,(H,13,14)
InChI key
GNUDAJTUCJEBEI-UHFFFAOYSA-N
Categorías relacionadas
Application
- To synthesize 9H-pyrimido[4,5-b]indole and aryl-benzimidazole based BET bromodomain and extra terminal (BET) protein inhibitors.
- To prepare naphthalimide based photo-exchangeable photochromic fluorescent molecules.
- As a reactant to develop DNA-encoded chemical libraries by palladium-catalyzed Suzuki coupling reaction with DNA-linked aryl halides.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Artículos
Suzuki-Miyaura cross-coupling reaction is extensively used in organic chemistry, polymer science, and pharmaceutical industries.
Suzuki-Miyaura cross-coupling reaction is extensively used in organic chemistry, polymer science, and pharmaceutical industries.
Suzuki-Miyaura cross-coupling reaction is extensively used in organic chemistry, polymer science, and pharmaceutical industries.
Suzuki-Miyaura cross-coupling reaction is extensively used in organic chemistry, polymer science, and pharmaceutical industries.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico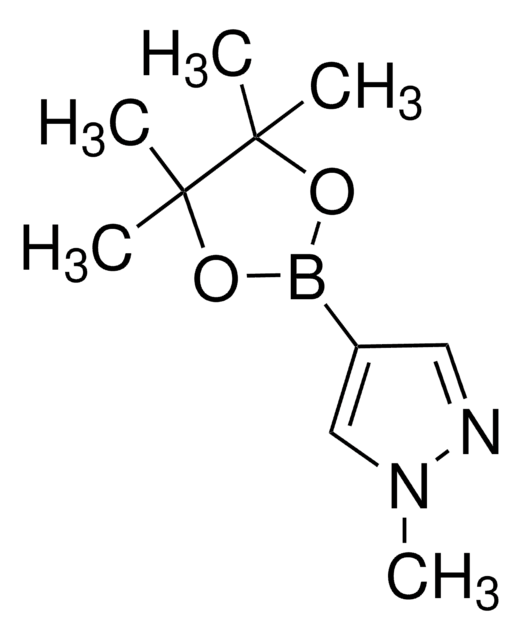

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)


![[1,1′-bis(difenilfosfino)ferroceno]dicloropaladio(II), complejo con diclorometano](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)