357774
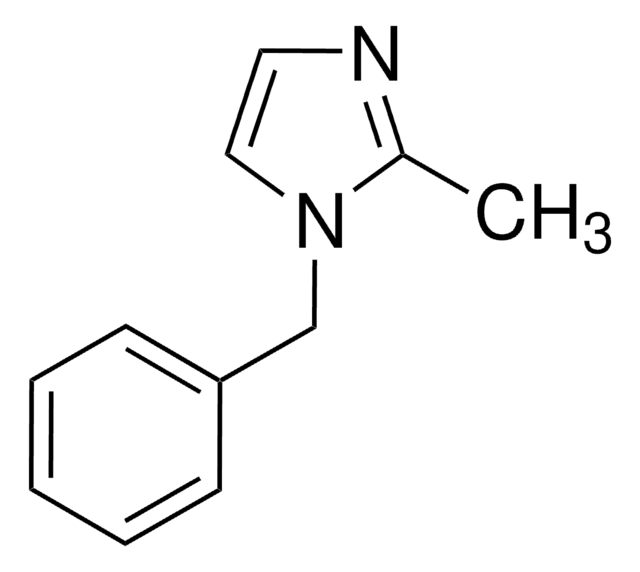
1-Phenylimidazole
97%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C9H8N2
Número de CAS:
Peso molecular:
144.17
Número MDL:
Código UNSPSC:
12352005
ID de la sustancia en PubChem:
NACRES:
NA.22
Productos recomendados
Ensayo
97%
Formulario
liquid
bp
142 °C/15 mmHg (lit.)
mp
13 °C (lit.)
densidad
1.14 g/mL at 25 °C (lit.)
cadena SMILES
c1ccc(cc1)-n2ccnc2
InChI
1S/C9H8N2/c1-2-4-9(5-3-1)11-7-6-10-8-11/h1-8H
Clave InChI
SEULWJSKCVACTH-UHFFFAOYSA-N
Descripción general
1-Phenylimidazole is an imidazole derivative. It induces 7-ethoxyresorufin-O-deethylase (EROD) activity in rainbow trout (Oncorhynchus mykiss) hepatocytes. The S(1)→S(0) transition of 1-phenylimidazole has been investigated in a supersonic jet expansion by resonant two-photon ionization. 1-Phenylimidazole is reported to be inhibitor of calmodulin-dependent nitric-oxide synthase from bovine brain and GHs pituitary cells.
Aplicación
1-Phenylimidazole is a suitable reagent used to investigate its effect on the citrulline formation by bovine brain nitric-oxide synthase.
Palabra de señalización
Warning
Frases de peligro
Clasificaciones de peligro
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órganos de actuación
Respiratory system
Código de clase de almacenamiento
10 - Combustible liquids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
235.4 °F - closed cup
Punto de inflamabilidad (°C)
113 °C - closed cup
Equipo de protección personal
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
A J Green et al.
Journal of biological inorganic chemistry : JBIC : a publication of the Society of Biological Inorganic Chemistry, 6(5-6), 523-533 (2001-07-27)
The bioI gene has been sub-cloned and over-expressed in Escherichia coli, and the protein purified to homogeneity. The protein is a cytochrome P450, as indicated by its visible spectrum (low-spin haem iron Soret band at 419 nm) and by the
A Jos et al.
Toxicology in vitro : an international journal published in association with BIBRA, 21(7), 1307-1310 (2007-05-25)
The classical pathway for induction of cytochrome P4501A (CYP1A) by xenobiotics is ligand binding to the aryl hydrocarbon receptor (AhR). However, several studies with mammalian cell systems point out a range of xenobiotics including imidazole derivatives, which are able to
M R Anari et al.
Chemical research in toxicology, 9(6), 924-931 (1996-09-01)
Organic hydroperoxides are believed to be primarily detoxified in cells by the GSH peroxidase/GSSG reductase system and activated to cytotoxic radical species by non-heme iron. However, organic hydroperoxides seem to be bioactivated by cytochrome P450 (P450) in isolated hepatocytes as
C F Wilkinson et al.
Biochemical pharmacology, 32(6), 997-1003 (1983-03-15)
Equilibrium dialysis studies established that 1-[4'-(3H)-phenyl]imidazole (PI) was bound to hepatic microsomal suspensions from control, phenobarbital (PB)- and 3-methylcholanthrene (3MC)-treated rats and that the binding was directly related to the cytochrome P-450 content. Computer-assisted Scatchard plot analysis of the binding
Filippo Monti et al.
Inorganic chemistry, 54(6), 3031-3042 (2015-03-06)
A series of cationic iridium(III) complexes with two carbene-based cyclometalating ligands and five different N^N bipyridine and 1,10-phenanthroline ancillary ligands is presented. For the first time--in the frame of a rarely studied class of bis(heteroleptic) iridium complexes with two carbene-based
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico











![1-[2-(Trifluoromethyl)phenyl]imidazole](/deepweb/assets/sigmaaldrich/product/structures/150/780/ea7e6b25-7659-422e-868c-8df7fd70d66e/640/ea7e6b25-7659-422e-868c-8df7fd70d66e.png)

