292710
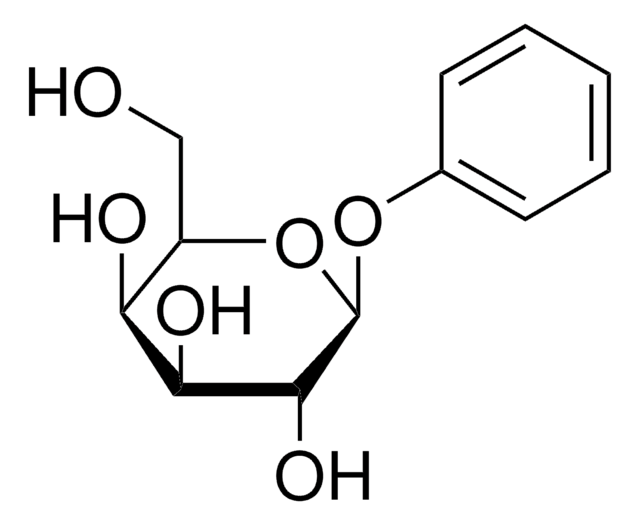
Phenyl β-D-glucopyranoside
≥95.0%
Sinónimos:
Phenyl beta-D-glucoside
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C12H16O6
Número de CAS:
Peso molecular:
256.25
Beilstein:
87517
Número CE:
Número MDL:
Código UNSPSC:
12352201
ID de la sustancia en PubChem:
NACRES:
NA.22
Productos recomendados
Nivel de calidad
Ensayo
≥95.0%
Formulario
powder
actividad óptica
[α]25/D −70°, c = 1 in H2O
mp
176-178 °C (lit.)
cadena SMILES
OC[C@H]1O[C@@H](Oc2ccccc2)[C@H](O)[C@@H](O)[C@@H]1O
InChI
1S/C12H16O6/c13-6-8-9(14)10(15)11(16)12(18-8)17-7-4-2-1-3-5-7/h1-5,8-16H,6H2/t8-,9-,10+,11-,12-/m1/s1
Clave InChI
NEZJDVYDSZTRFS-RMPHRYRLSA-N
Aplicación
Phenyl β-D-glucopyranoside can be used:
- As a starting material for the synthesis of various derivatives of β-D-glucopyranosides with potential application as anti-HIV agents.
- As a model for glycosides in the gas phase for their spectroscopic investigation.
- As an internal standard in GC and GC-MS quantitative analyses.
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
4-(Arylamino)phenyl alpha-D-glucopyranosides as potential anti-HIV agents.
J C Briggs et al.
Carbohydrate research, 282(2), 293-298 (1996-03-18)
Sugars in the gas phase: the spectroscopy and structure of jet-cooled phenyl ?-D-glucopyranoside.
Talbot FO and Simons JP
Physical Chemistry Chemical Physics, 4(15), 3562-3565 (2002)
Chunyan Bao et al.
Carbohydrate research, 339(7), 1311-1316 (2004-04-29)
A new hydrogel based on a substituted phenyl glucoside with a Schiff base in the aglycon was synthesized, and the self-assembling characteristics was studied. FTIR spectra, UV-vis absorption spectra and X-ray diffraction (XRD) revealed that pi-pi interactions between the Schiff
Ying Na et al.
Bioorganic chemistry, 39(3), 111-113 (2011-03-26)
The spontaneous hydrolysis of glycosylamines, where the aglycone is either a primary amine or ammonia, is over a hundred million-times faster than that of O- or S-glycosides. The reason for this (as pointed out by Capon and Connett in 1965)
V T Edwards et al.
Xenobiotica; the fate of foreign compounds in biological systems, 16(9), 801-807 (1986-09-01)
The metabolic fates of 14C-phenol and its model plant conjugates 14C-phenyl glucoside and 14C-phenyl 6-O-malonyl-glucoside have been compared following equimolar oral dosing to rats (1.2 mg phenol/kg). Rapid excretion of radioactivity in the urine (at least 80% within 24 h)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico