115819
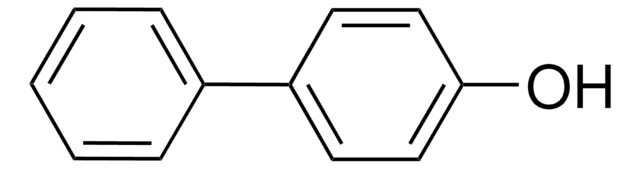
2,2′-Biphenol
99%
Sinónimos:
2,2′-Biphenyldiol, 2,2′-Dihydroxybiphenyl, 2,2′-Diphenol
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
HOC6H4C6H4OH
Número de CAS:
Peso molecular:
186.21
Beilstein:
1638363
Número CE:
Número MDL:
Código UNSPSC:
12162002
ID de la sustancia en PubChem:
NACRES:
NA.23
Productos recomendados
Nivel de calidad
Ensayo
99%
Formulario
solid
bp
315 °C (lit.)
mp
108-110 °C (lit.)
cadena SMILES
Oc1ccccc1-c2ccccc2O
InChI
1S/C12H10O2/c13-11-7-3-1-5-9(11)10-6-2-4-8-12(10)14/h1-8,13-14H
Clave InChI
IMHDGJOMLMDPJN-UHFFFAOYSA-N
Categorías relacionadas
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 2
Punto de inflamabilidad (°F)
309.2 °F - closed cup - (External MSDS)
Punto de inflamabilidad (°C)
154 °C - closed cup - (External MSDS)
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Sierra Rayne et al.
Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology, 4(11), 876-886 (2005-10-28)
Photochemical studies on a range of model dibenzo[1,4]dioxins were performed in aqueous and organic solutions. The compounds were found to undergo a photochemically initiated aryl-ether bond homolysis that yields reactive 2-spiro-6'-cyclohexa-2',4'-dien-1'-one and subsequent 2,2'-biphenylquinone intermediates. Under steady-state irradiation, the 2,2'-biphenylquinones
Bernd Schmidt et al.
The Journal of organic chemistry, 78(17), 8680-8688 (2013-08-01)
User-friendly protocols for the protecting group-free synthesis of 2,2'-biphenols via Suzuki-Miyaura coupling of o-halophenols and o-boronophenol are presented. The reactions proceed in water in the presence of simple additives such as K2CO3, KOH, KF, or TBAF and with commercially available
R J Heath et al.
The Journal of biological chemistry, 273(46), 30316-30320 (1998-11-07)
The broad spectrum antibacterial properties of 2-hydroxydiphenyl ethers have been appreciated for decades, and their use in consumer products is rapidly increasing. We identify the enoyl-acyl carrier protein reductase (fabI) component of the type II fatty acid synthase system as
W A Prütz et al.
International journal of radiation biology and related studies in physics, chemistry, and medicine, 44(2), 183-196 (1983-08-01)
Phenoxyl radicals generated pulse radiolytically by the reaction of N.3 with Gly-Tyr decay biomolecularly (2k = 4.7 X 10(8)M-1 s-1) with efficient formation of 2,2'-dimers, which enolize rapidly (k = 2.7 X 10(4) s-1) to produce the 2,2'-biphenolic product. The
Christina DiMarco-Crook et al.
Journal of food science, 85(4), 1292-1301 (2020-03-08)
Chemoprevention strategies employing the use of multiple dietary bioactive components and their metabolites in combination offer advantages due to their low toxicity and potential synergistic interactions. Herein, for the first time, we studied the combination of curcumin and 3',4'-didemethylnobiletin (DDMN)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico