B25606
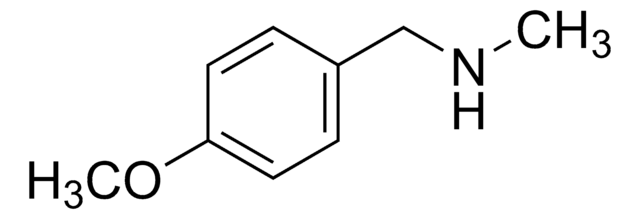
N-Benzylmethylamine
97%
Synonym(s):
N-Methylbenzylamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5CH2NHCH3
CAS Number:
Molecular Weight:
121.18
Beilstein:
606221
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.522 (lit.)
bp
184-189 °C (lit.)
density
0.939 g/mL at 25 °C (lit.)
SMILES string
CNCc1ccccc1
InChI
1S/C8H11N/c1-9-7-8-5-3-2-4-6-8/h2-6,9H,7H2,1H3
InChI key
RIWRFSMVIUAEBX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1 - Skin Corr. 1B - Skin Sens. 1
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
167.0 °F - closed cup
Flash Point(C)
75 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Steven Droge et al.
Environmental science & technology, 46(11), 5894-5901 (2012-05-01)
Sorption of organic cations to soil organic matter was studied using dynamic column experiments with different compositions of electrolytes in aqueous eluents. The sorption affinity of the tested variety of charged compounds, including primary, secondary, and tertiary amines and quaternary
Zinc deficiency and the induction of oesophageal tumors in rats by benzylmethylamine and sodium nitrite.
L Y Fong et al.
IARC scientific publications, (41)(41), 679-683 (1982-01-01)
T Tahira et al.
Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 26(6), 511-516 (1988-06-01)
Thioproline, which is readily nitrosated to form nitrosothioproline, is expected to act as a nitrite scavenger. The effect of thioproline as an inhibitor of the carcinogenesis induced by N-nitroso-N-benzylmethylamine precursors was examined. Two groups of male F-344 rats were given
R Siegler et al.
Journal of pharmaceutical and biomedical analysis, 7(1), 45-55 (1989-01-01)
DTAF has been used successfully to prepare fluorescent labelled reagents for fluorescence polarization immunoassays. Its applicability as a derivation reagent for direct fluorescence analysis of primary and secondary amines was evaluated. DTAF was shown to have spectral properties that closely
H Weber et al.
Journal of chromatography, 307(1), 145-153 (1984-04-13)
The separation of racemic benoxaprofen into the two benoxaprofen enantiomers by preparative high-performance liquid chromatography and the application of the activated enantiomers as derivatization reagents for the simultaneous stereoselective determination of chiral amines in biological material is described. Activated (+)-
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service