169021
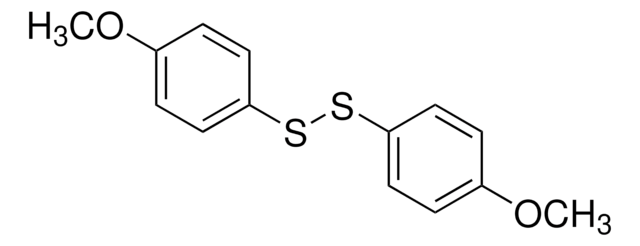
Phenyl disulfide
99%
Synonym(s):
Diphenyl disulfide, NSC 2689
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Linear Formula:
C6H5SSC6H5
CAS Number:
Molecular Weight:
218.34
Beilstein:
639794
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
form:
powder
Assay:
99%
Recommended Products
Quality Level
Assay
99%
form
powder
mp
58-60 °C (lit.)
solubility
xylene: soluble 3%, clear, colorless to yellow
functional group
disulfide
SMILES string
S(Sc1ccccc1)c2ccccc2
InChI
1S/C12H10S2/c1-3-7-11(8-4-1)13-14-12-9-5-2-6-10-12/h1-10H
InChI key
GUUVPOWQJOLRAS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Phenyl disulfide is used as a precursor for the synthesis of phenyl selenosulfide (PhS-SePh), which is vital in Li-ion battery production.
Application
Phenyl disulfide is the hydrolysis product of dyfonate( insecticide). Phenyl disulfide (diphenyl disulphide) participates in hydrothiolation of alkynes via amine-mediated single electron transfer mechanism.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Mixture is better: enhanced electrochemical performance of phenyl selenosulfide in rechargeable lithium batteries
Guo, Wei and Bhargav
Journal of the Chemical Society. Chemical Communications, 54, 8873-8876 (2018)
Identification of hydrolytic metabolites of dyfonate in alkaline aqueous solutions by using high performance liquid chromatography-UV detection and gas chromatography-mass spectrometry.
Wang T, et al.
International Journal of Environmental Analytical Chemistry, 90(12), 948-961 (2010)
Molecular modeling and enzyme kinetics indicate a novel mechanism for mammalian 5-lipoxygenase.
R W Egan et al.
Advances in prostaglandin, thromboxane, and leukotriene research, 17A, 69-74 (1987-01-01)
C W Nogueira et al.
Toxicology, 191(2-3), 169-178 (2003-09-11)
Organochalcogens are important intermediates and useful reagents in organic synthesis, which can increase human exposure risk to these chemicals in the workplace. As well, there are a number of reported cases of acute toxicity following organochalcogen ingestion of vitamins and
J M Young et al.
Agents and actions, 21(3-4), 314-315 (1987-08-01)
Indomethacin was administered subcutaneously to rats, 4 mg/kg/day for 4 consecutive days in order to produce erosions of the small intestine which were scored at necropsy on day 5. Orally administered phenidone (up to 250 mg/kg/day), a mixed cycloocygenase-lipoxygenase inhibitor
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service