T1633
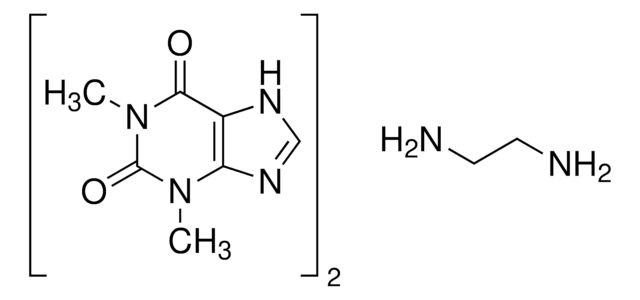
Theophylline
≥99% (HPLC), powder, phosphodiesterase inhibitor
Synonym(s):
1,3-Dimethylxanthine, 2,6-Dihydroxy-1,3-dimethylpurine, 3,7-Dihydro-1,3-dimethyl-1H-purine-2,6-dione
About This Item
Recommended Products
product name
Theophylline, anhydrous, ≥99%, powder
grade
anhydrous
Assay
≥99%
form
powder
color
white
solubility
H2O: slightly soluble 8.3 mg/ml
NH4OH: 50 mg/ml, clear, colorless
alcohol: 12.5 mg/ml
chloroform: soluble 9.1 mg/ml
0.1 M HCl: soluble
0.1 M NaOH: soluble
ammonium hydroxide: soluble
aqueous base: soluble
diethyl ether: slightly soluble
dilute HCl: soluble
dilute nitric acid: soluble
ethanol: moderately soluble
originator
Forest Labs
SMILES string
CN1C(=O)N(C)c2[nH]cnc2C1=O
InChI
1S/C7H8N4O2/c1-10-5-4(8-3-9-5)6(12)11(2)7(10)13/h3H,1-2H3,(H,8,9)
InChI key
ZFXYFBGIUFBOJW-UHFFFAOYSA-N
Gene Information
human ... ADORA1(134) , ADORA2A(135) , ADORA2B(136) , ADORA3(140) , PDE3A(5139) , PDE3B(5140) , PDE4A(5141) , PDE4B(5142) , PDE4C(5143) , PDE4D(5144)
mouse ... Adora2b(11541)
rat ... Adora1(29290) , Adora2a(25369) , Adora2b(29316) , Pde1b(29691) , Pde3a(50678)
Looking for similar products? Visit Product Comparison Guide
Application
Biochem/physiol Actions
Features and Benefits
Preparation Note
The solubility of the methylxanthines is low, but can be enhanced by the formation of complexes (usually 1:1) with a wide variety of compounds such as ethylenediamine (to form aminophylline). The formation of complex double salts (caffeine and sodium benzoate) or true salts (like choline theophyllinate, and oxtriphylline) also improves aqueous solubility. These salts or complexes dissociate to yield the parent methylxanthines when dissolved in biological fluids and should not be confused with covalently modified derivatives such asdyphylline (1,3-dimethyl-7-(2,3-dihydroxypropyl)-xanthine).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Repr. 1B
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Cyclic nucleotides like cAMP modulate cell function via PKA activation and ion channels.
Cyclic nucleotides like cAMP modulate cell function via PKA activation and ion channels.
Cyclic nucleotides like cAMP modulate cell function via PKA activation and ion channels.
Cyclic nucleotides like cAMP modulate cell function via PKA activation and ion channels.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service