691399
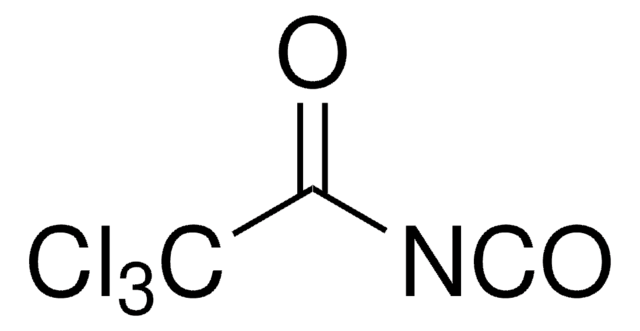
Chlorosulfonyl isocyanate
Arxada quality, 99.0-100.3 % (w/w) (T)
Synonym(s):
N-Carbonylsulfamyl chloride, CSI
About This Item
Recommended Products
vapor pressure
5.57 psi ( 20 °C)
Quality Level
form
liquid
quality
Arxada quality
manufacturer/tradename
Arxada AG
concentration
99.0-100.3 % (w/w) (T)
impurities
≤1.00% chloropropylsulfonyl isocyanate
refractive index
n20/D 1.447 (lit.)
bp
107 °C (lit.)
mp
−44 °C (lit.)
density
1.626 g/mL at 25 °C (lit.)
SMILES string
ClS(=O)(=O)N=C=O
InChI
1S/CClNO3S/c2-7(5,6)3-1-4
InChI key
WRJWRGBVPUUDLA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Pharmaceutical intermediates: A study explored the synthesis and carbonic anhydrase inhibitory properties of sulfamides derived from Chlorosulfonyl isocyanate, demonstrating its potential in the development of new therapeutic agents, particularly for treating conditions involving carbonic anhydrase enzymes (Aksu et al., 2013).
- Stereochemical synthesis applications: Chlorosulfonyl isocyanate was utilized for the stereoselective amination of chiral benzylic ethers in the total synthesis of (+)-sertraline, showcasing its critical role in the precise construction of complex pharmaceuticals (Lee et al., 2011).
- One-pot synthetic transformations: The compound′s use in one-pot conversion of trimethylsilyl ethers into urethanes was highlighted, further applied to synthesize novel neuromodulators like carisbamate. This illustrates Chlorosulfonyl isocyanate′s versatility in facilitating efficient and innovative synthetic routes in medicinal chemistry (Dong et al., 2008).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Resp. Sens. 1 - Skin Corr. 1B - Skin Sens. 1
Supplementary Hazards
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
>230.0 °F
Flash Point(C)
> 110 °C
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service