570656
5-Vinyluracil
95%
Synonym(s):
5-Vinyl-1H-pyrimidine-2,4-dione
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H6N2O2
CAS Number:
Molecular Weight:
138.12
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
solid
mp
>300 °C (dec.) (lit.)
SMILES string
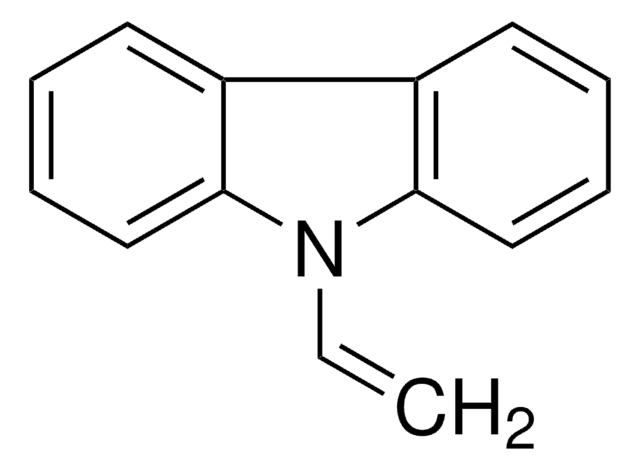
C=CC1=CNC(=O)NC1=O
InChI
1S/C6H6N2O2/c1-2-4-3-7-6(10)8-5(4)9/h2-3H,1H2,(H2,7,8,9,10)
InChI key
ZRYZBEQILKESAW-UHFFFAOYSA-N
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
E T Chelton et al.
The Biochemical journal, 187(1), 257-260 (1980-04-01)
Bacteriophage T3 was produced in a form that contained 32% of its normal DNA thymine residues replaced with 5-vinyluracil residues by infecting a thymine-requiring strain of Escherichia coli with phage T3 in a medium containing 5-vinyluracil. When 2'-deoxy-5-vinyluridine was added
A S Jones et al.
Nucleic acids research, 1(1), 105-107 (1974-01-01)
A method for the rapid preparation of the thymineanalogue, 5-vinyluracil, in 83% yield from 5-(1-hydroxyethyl)uracil via the methanesulphonyl ester is reported.
Kazuo Hattori et al.
Bioorganic & medicinal chemistry letters, 13(5), 867-872 (2003-03-06)
A series of tumor-activated prodrugs of the inhibitors of dihydropyrimidine dehydrogenase (DPD), an enzyme catabolizing 5-fluorouracil (5-FU: 4g), has been designed and synthesized. RO0094889 (11c) is a prodrug of 5-vinyluracil (4c), a known DPD inhibitor, and was designed to generate
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service