298891
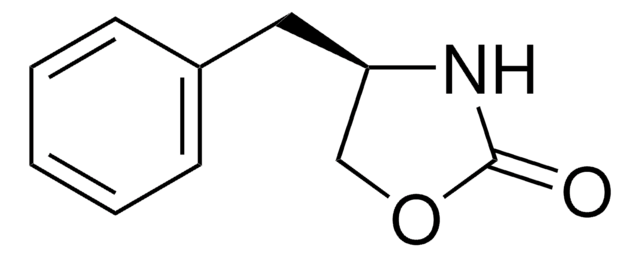
(4R,5S)-(+)-4-Methyl-5-phenyl-2-oxazolidinone
99%
Synonym(s):
(4R,5S)-4-Methyl-5-phenyl-2-oxazolidinone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H11NO2
CAS Number:
Molecular Weight:
177.20
Beilstein:
1211705
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
optical activity
[α]18/D +168°, c = 2 in chloroform
optical purity
ee: 98% (GLC)
mp
121-123 °C (lit.)
SMILES string
C[C@H]1NC(=O)O[C@H]1c2ccccc2
InChI
1S/C10H11NO2/c1-7-9(13-10(12)11-7)8-5-3-2-4-6-8/h2-7,9H,1H3,(H,11,12)/t7-,9-/m1/s1
InChI key
PPIBJOQGAJBQDF-VXNVDRBHSA-N
Application
Evan′s chiral auxiliary (4R,5S)-(+)-4-Methyl-5-phenyl-2-oxazolidinone reacts with carboxylic acids to produce corresponding acyl derivatives in the presence of a diisopropylcarbodiimide reagent. It can also employed in the preparation of N-sulfinyloxazolidinone reagent (chiral sulfinyl transfer reagent), which reacts with nucleophiles such as Grignard reagents, enolates, and metalated amides to produce the chiral sulfoxides, sulfinate esters, and sulfonamides.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
DIC-mediated coupling of carboxylic acids to (4R, 5S)-4-methyl-5-phenyl-2-oxazolidinone
Graham JM, et al.
Synthetic Communications, 30(7), 1221-1226 (2000)
The Journal of Organic Chemistry, 57, 1744-1744 (1992)
Tetrahedron, 48, 7527-7527 (1992)
Journal of the American Chemical Society, 115, 10742-10742 (1993)
The Journal of Organic Chemistry, 58, 2725-2725 (1993)
Chromatograms
application for HPLCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service