36928
1,2,3,5-Tetrachlorobenzene
PESTANAL®, analytical standard
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
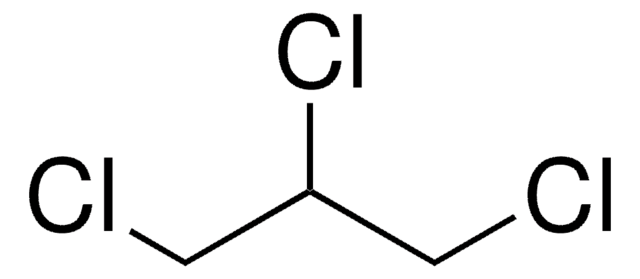
C6H2Cl4
CAS Number:
Molecular Weight:
215.89
Beilstein:
1618864
EC Number:
MDL number:
UNSPSC Code:
41116107
PubChem Substance ID:
NACRES:
NA.24
Recommended Products
grade
analytical standard
Quality Level
product line
PESTANAL®
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
agriculture
environmental
format
neat
SMILES string
Clc1cc(Cl)c(Cl)c(Cl)c1
InChI
1S/C6H2Cl4/c7-3-1-4(8)6(10)5(9)2-3/h1-2H
InChI key
QZYNWJQFTJXIRN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
Recommended products
Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
Legal Information
PESTANAL is a registered trademark of Merck KGaA, Darmstadt, Germany
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
W De Wolf et al.
Comparative biochemistry and physiology. C, Comparative pharmacology and toxicology, 100(1-2), 55-57 (1991-01-01)
1. Seven chlorinated anilines and one chlorinated benzene were tested for their ability to bioconcentrate in guppies (Poecilia reticulata) under different experimental conditions. 2. Interactions between compounds in a mixture influence the bioconcentration of some chlorinated anilines. These interactions result
M Parrini et al.
Bollettino della Societa italiana di biologia sperimentale, 66(7), 709-716 (1990-07-01)
The mutagenicity of halogenated benzenes, including three isomers of tri- and tetrachlorobenzenes (TCB, TeCB) was studied on male Swiss CD1 mice by MN test. The data presented show that all the halogenated benzenes tested were found to be clastogenic apart
Mei Sun et al.
Environmental science & technology, 44(21), 8209-8215 (2010-10-01)
Sediment caps that degrade contaminants can improve their ability to contain contaminants relative to sand and sorbent-amended caps, but few methods to enhance contaminant degradation in sediment caps are available. The objective of this study was to determine if, carbon
Peter Kämpfer et al.
International journal of systematic and evolutionary microbiology, 54(Pt 3), 749-751 (2004-05-15)
A Gram-positive, rod-shaped, non-spore-forming bacterium (B5(T)) was isolated from an enrichment culture that contained 1,2,3,5-tetrachlorobenzene as the sole source of carbon. On the basis of 16S rRNA gene sequence similarity studies, strain B5(T) was shown to belong to the family
Koji Ito et al.
Journal of pesticide science, 44(3), 171-176 (2019-09-19)
The substrate range of Nocardioides sp. strain PD653, capable of mineralizing hexachlorobenzene, was investigated based on the dissipation of substrates and the liberation of halogen ions. Strain PD653 dehalogenated 10 out of 18 halophenol congeners; however, it could dehalogenate only
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service