All Photos(1)
About This Item
Empirical Formula (Hill Notation):
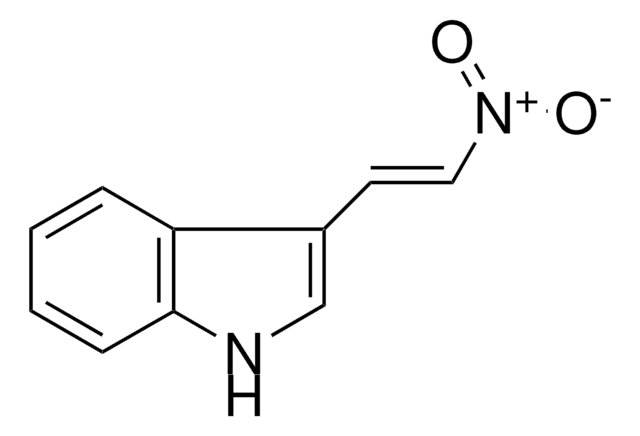
C6H5NO3
CAS Number:
Molecular Weight:
139.11
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
72-75 °C (lit.)
SMILES string
[O-][N+](=O)\C=C\c1ccco1
InChI
1S/C6H5NO3/c8-7(9)4-3-6-2-1-5-10-6/h1-5H/b4-3+
InChI key
WVUICGOYGDHVBH-ONEGZZNKSA-N
Related Categories
General description
2-(2-Nitrovinyl)furan (NVF) is a 2-furylethylene derivative with a potential antiprotozoal property. It forms solid inclusion complexes with cyclodextrine (CD) derivatives, 2-hydroxypropyl-β-cyclodextrin and sulfobutyl ether-β-cyclodextrin. The Friedel-Crafts alkylation of naphthols with NVF in aqueous medium and catalyst-free condition has been reported. The formation of Morita-Baylis-Hillman (MBH) adduct via MBH reaction of NVF with diisopropyl azodicarboxylate (DIAD) has been studied.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Diana Oliveira et al.
International journal of molecular sciences, 22(2) (2021-01-14)
Quorum sensing (QS) plays an essential role in the production of virulence factors, in biofilm formation and antimicrobial resistance. Consequently, inhibiting QS is being considered a promising target for antipathogenic/anti-virulence therapies. This study aims to screen 2-nitrovinylfuran derivatives structurally related
Characterization and molecular modeling of the inclusion complexes of 2-(2-nitrovinyl) furan (G-0) with cyclodextrines.
Ruz V, et al.
International Journal of Pharmaceutics, 439(1), 275-285 (2012)
T O Ajiboye et al.
Microbial pathogenesis, 91, 107-114 (2015-12-02)
The involvement of reactive oxygen species and oxidative stress in 2-(2-nitrovinyl) furan mediated bacterial cell death was investigated in Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. Time kill assay resulted in significant decrease in the optical density and colony-forming unit
Catalyst-free Friedel-Crafts alkylation of naphthols with nitrostyrenes in the presence of water.
Halimehjani AZ, et al.
Tetrahedron Letters, 50(13), 1441-1443 (2009)
Highly efficient hydrazination of conjugated nitroalkenes via imidazole or DMAP mediated Morita-Baylis-Hillman reaction.
Dadwal M, et al.
Organic & Biomolecular Chemistry, 4(13), 2525-2528 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service