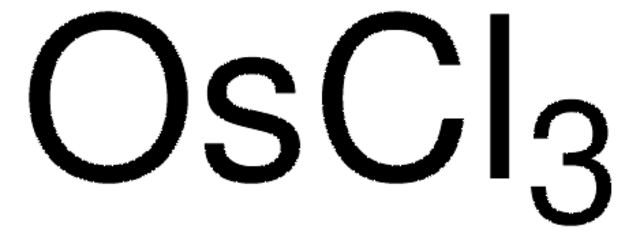All Photos(2)
About This Item
Empirical Formula (Hill Notation):
Os
CAS Number:
Molecular Weight:
190.23
EC Number:
MDL number:
UNSPSC Code:
11101711
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
99.9% trace metals basis
form
powder
resistivity
8.12 μΩ-cm, 20°C
bp
5027 °C (lit.)
mp
3045 °C (lit.)
density
22.61 g/cm3 (lit.)
SMILES string
[Os]
InChI
1S/Os
InChI key
SYQBFIAQOQZEGI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Flam. Sol. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
nwg
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Hideki Sugimoto et al.
Journal of the American Chemical Society, 134(46), 19270-19280 (2012-11-02)
For the synthesis of the 1,2-diols, cis-1,2-dihydroxylation of alkenes catalyzed by osmium(VIII) tetroxide (OsO(4)) is a powerful method. However, OsO(4) is quite toxic due to its highly volatile and sublimable nature. Thus, the development of alternative catalysts for cis-1,2-dihydroxylation of
Carole J R Bataille et al.
Chemical Society reviews, 40(1), 114-128 (2010-11-05)
Numerous synthetic protocols for producing syn-diols from the corresponding alkenes have been developed and published over recent years. It is the intent of the following tutorial review to present a concise summary of the main methods used to prepare syn-diol
Vladimir B Arion et al.
Chemical communications (Cambridge, England), 48(68), 8559-8561 (2012-07-17)
A modified paullone ligand bearing a TEMPO free-radical unit (HL) and its ruthenium(II) and osmium(II)-arene complexes [M(p-cymene)(HL)Cl]Cl·nH(2)O (M = Ru, Os) exhibit high antiproliferative activity in human cancer cell lines.
Gabriel E Büchel et al.
Journal of inorganic biochemistry, 113, 47-54 (2012-06-13)
A one-pot synthesis of osmium(IV) complexes with two different tautomers of indazole, 1H-indazole and 2H-indazole, namely (H(2)ind)[Os(IV)Cl(5)(2H-ind)] (1) and (H(2)ind)[Os(IV)Cl(5)(1H-ind)] (2) is reported. Both compounds have been comprehensively characterized by NMR spectroscopy, ESI (electrospray ionization) mass spectrometry, electronic absorption spectroscopy
Werner Ginzinger et al.
Journal of medicinal chemistry, 55(7), 3398-3413 (2012-03-16)
A series of ruthenium(II) arene complexes with 3-(1H-benzimidazol-2-yl)-1H-quinoxalin-2-one, bearing pharmacophoric groups of known protein kinase inhibitors, and related benzoxazole and benzothiazole derivatives have been synthesized. In addition, the corresponding osmium complexes of the unsubstituted ligands have also been prepared. The
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service