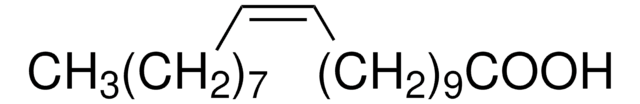43051
Palmitic acid
certified reference material, TraceCERT®, Manufactured by: Sigma-Aldrich Production GmbH, Switzerland
Synonym(s):
1-Pentadecanecarboxylic acid, C16:0, Cetylic acid, Hexadecanoic acid, NSC 5030, PamOH
About This Item
Recommended Products
grade
certified reference material
TraceCERT®
Quality Level
vapor pressure
10 mmHg ( 210 °C)
product line
TraceCERT®
shelf life
limited shelf life, expiry date on the label
manufacturer/tradename
Manufactured by: Sigma-Aldrich Production GmbH, Switzerland
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
bp
271.5 °C/100 mmHg (lit.)
mp
61-62.5 °C (lit.)
density
0.852 g/mL at 25 °C (lit.)
application(s)
cleaning products
cosmetics
food and beverages
personal care
format
neat
functional group
carboxylic acid
shipped in
ambient
storage temp.
20-25°C
SMILES string
CCCCCCCCCCCCCCCC(O)=O
InChI
1S/C16H32O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16(17)18/h2-15H2,1H3,(H,17,18)
InChI key
IPCSVZSSVZVIGE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Certified content by quantitative NMR incl. uncertainty and expiry date are given on the certificate.
Download your certificate at: http://www.sigma-aldrich.com.
Application
Packaging
Legal Information
Storage Class Code
11 - Combustible Solids
WGK
nwg
Flash Point(F)
235.4 °F
Flash Point(C)
113 °C
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service