553603
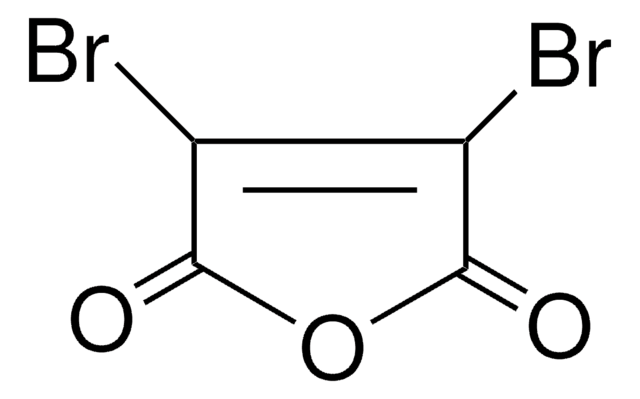
2,3-Dibromomaleimide
97%
Synonym(s):
3,4-Dibromopyrrole-2,5-dione
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C4HBr2NO2
CAS Number:
Molecular Weight:
254.86
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
228-231 °C (lit.)
SMILES string
BrC1=C(Br)C(=O)NC1=O
InChI
1S/C4HBr2NO2/c5-1-2(6)4(9)7-3(1)8/h(H,7,8,9)
InChI key
BIKSKRPHKQWJCW-UHFFFAOYSA-N
General description
2,3-Dibromomaleimide a cyclic imide, has a dipole moment of 0.30 debye based on ab initio and density functional theory (DFT) calculations.
Application
2,3-Dibromomaleimide may be used in the synthesis of the following:
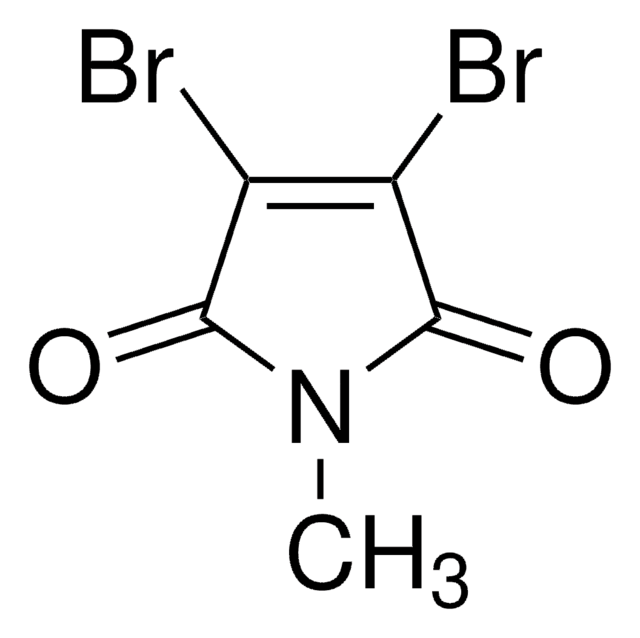
- 2-bromo-3-(1H-indol-3-yl)maleimide via reaction with indolyl magnesium bromide in tetrahydrofuran
- 2-dibromo-3-propylsulfanyl-maleimide via reaction with propanethiol and sodium acetate in methanol
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B - Skin Sens. 1
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A method for preparing water soluble cyclic polymers
Long S, et al.
Reactive functional Polymers, 80, 15-20 (2014)
Strong fluorescence enhancement of 2-bromo-3-(1H-indol-3-yl) maleimide upon coordination to a Lewis-acidic metal complex.
Kaletas BK, et al.
Chemical Communications (Cambridge, England), 7, 776-777 (2002)
Polarizability and first-order hyperpolarizability of cyclic imides.
Asghari-Khiavi M, et al.
Journal of Molecular Structure: THEOCHEM, 910(1), 56-60 (2009)
Jordon Sandland et al.
Bioconjugate chemistry, 30(4), 975-993 (2019-02-16)
This Review aims to highlight key aspects of tetrapyrrole-based antibody-drug conjugates (ADCs) and significant developments in the field since 2010. Many new conjugation methods have been developed and employed in the past decade, and associated with this, there has been
Ting Bai et al.
Macromolecular bioscience, 20(7), e1900438-e1900438 (2020-05-15)
Targeting the distinct cholesterol metabolism of tumor cells is proposed as a novel way to treat tumors. Blocking acyl-CoA cholesterol acyltransferase-1 (ACAT-1) by the inhibitor avasimible (Ava), which elevates intracellular free cholesterol levels, is shown to effectively induce apoptosis. However
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service