55088
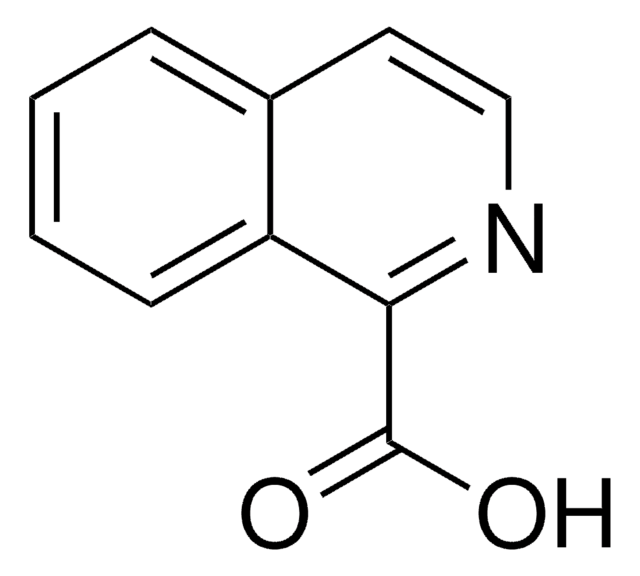
8-Hydroxy-2-quinolinecarboxylic acid
≥98.0% (HPLC)
Synonym(s):
8-Hydroxyquinaldic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H7NO3
CAS Number:
Molecular Weight:
189.17
Beilstein:
146082
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98.0% (HPLC)
form
solid
SMILES string
OC(=O)c1ccc2cccc(O)c2n1
InChI
1S/C10H7NO3/c12-8-3-1-2-6-4-5-7(10(13)14)11-9(6)8/h1-5,12H,(H,13,14)
InChI key
UHBIKXOBLZWFKM-UHFFFAOYSA-N
Related Categories
General description
8-Hydroxy-2-quinolinecarboxylic acid (8HQC) is a tridentate chelating agent. It reacts with 2-aminophenol to form a benzoxazole derivative, which can undergo fluorescence quenching in water, making it a viable candidate for developing a probe for detecting water in aprotic organic solvents. The analysis of mid-IR and Raman spectra of 8HQC shows the presence of seven tautomers.
Application
8-Hydroxy-2-quinolinecarboxylic acid may be used in the preparation of bis (8-hydroxyquinoline-2-carboxylato-Κ3N,O,O′) cobalt (II) trihydrate.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Katarzyna Walczak et al.
Molecules (Basel, Switzerland), 25(7) (2020-04-09)
8-Hydroxyquinaldic acid, the end-metabolite of tryptophan, is well-known metal chelator; however, its role in humans, especially in cancer promotion and progression, has not been fully revealed. Importantly, 8-hydroxyquinaldic acid is the analog of kynurenic acid with evidenced antiproliferative activity towards
A combined experimental and theoretical study on 8-hydroxy-2-quinolinecarboxylic acid.
Badoglu S and Yurdakul S.
Optics and Spectroscopy, 116(2), 196-206 (2014)
Determination of water content in aprotic organic solvents using 8-hydroxyquinoline based fluorescent probe.
Kim JS, et al.
Bull. Korean Chem. Soc., 27(12), 2058-2058 (2006)
Bis (8-hydroxyquinoline-2-carboxylato-?3N,O,O') cobalt (II) trihydrate.
Okabe N and Muranishi Y.
Acta Crystallographica Section E, Structure Reports Online, 58(7), m352-m353 (2002)
Francesco Bellia et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 26(70), 16690-16705 (2020-07-07)
Metal dysregulation, oxidative stress, protein modification, and aggregation are factors strictly interrelated and associated with neurodegenerative pathologies. As such, all of these aspects represent valid targets to counteract neurodegeneration and, therefore, the development of metal-binding compounds with other properties to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service