All Photos(1)
About This Item
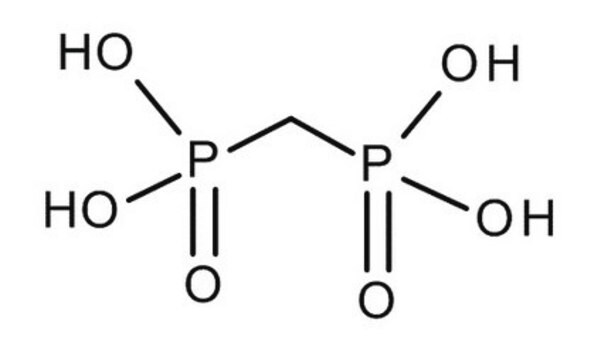
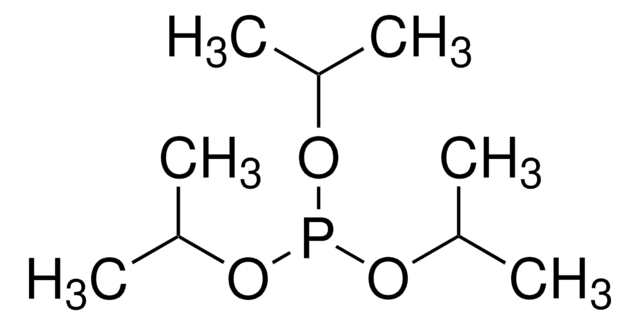
Linear Formula:
CH2[P(O)[OCH(CH3)2]2]2
CAS Number:
Molecular Weight:
344.32
Beilstein:
1080180
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.431 (lit.)
bp
155 °C/0.5 mmHg (lit.)
density
1.08 g/mL at 25 °C (lit.)
functional group
phosphonate
SMILES string
CC(C)OP(=O)(CP(=O)(OC(C)C)OC(C)C)OC(C)C
InChI
1S/C13H30O6P2/c1-10(2)16-20(14,17-11(3)4)9-21(15,18-12(5)6)19-13(7)8/h10-13H,9H2,1-8H3
InChI key
ODTQUKVFOLFLIQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Tetraisopropyl methylenediphosphonate (IPCP) is a tetraisopropyl ester of methylenediphosphonic acid. It is an electroneutral ligand, reported to be synthesized from triisopropyl phosphate. Powder electron paramagnetic resonance (EPR) studies of tris complexes of Cu(II) complexes with IPCP has been analyzed.
Application
Tetraisopropyl methylenediphosphonate [T(iPr)MDP] forms a synergistic mixture with hydrogen dicarbollylcobaltate in nitrobenzene which was used in the extraction of europium and americium.
It may be used in the synthesis of the following:
It may be used in the synthesis of the following:
- tetraisopropyl ethenylidenebisphosphonate.
- tetraisopropyl 4-acetylthiobutane-1,1-diphosphonate
- tetraisopropyl 2-(3,5-bis(bromomethyl)phenyl)ethane-1,1-diphosphonate
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
320.0 °F - closed cup
Flash Point(C)
160 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of Tetraisopropyl Methyenediphosphonate.
Jian-ming XU.
Jing Xi Hua Gong Zhong Jian Ti / Fine Chemical Intermediates, 4, 007-007 (2002)
Electron paramagnetic resonance studies of some copper (II) complexes with organophosphorus chelates.
Joesten MD, et al.
Journal of the American Chemical Society, 93(5), 1138-1140 (1971)
L Gil et al.
Bioorganic & medicinal chemistry, 7(5), 901-919 (1999-07-10)
Conjugates of bisphosphonates (potential bone resorption inhibitors) and prostaglandin E2 (a bone formation enhancer) were prepared and evaluated for their ability to bind to bone and to liberate, enzymatically, free PGE2. The conjugate 3, an amide at C-1 of PGE2
Synthesis of novel bisphosphonate polyamine conjugates and their affinity to hydroxyapatite.
Sankala E, et al.
ARKIVOC (Gainesville, FL, United States), 4, 233-241 (2012)
Extraction of europium and americium into nitrobenzene by using synergistic mixture of hydrogen dicarbollylcobaltate and tetraisopropyl methylene diphosphonate.
Makrlik E, et al.
J. Radioanal. Nucl. Chem., 283(3), 727-733 (2010)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)