All Photos(1)
About This Item
Empirical Formula (Hill Notation):
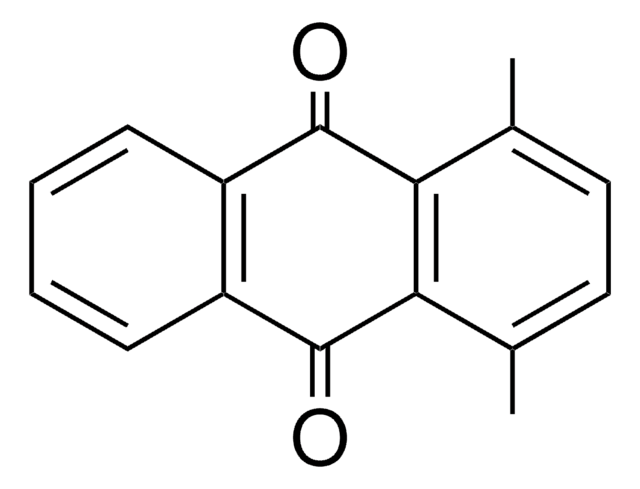
C16H12O2
CAS Number:
Molecular Weight:
236.27
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
210-212 °C (lit.)
SMILES string
Cc1cc2C(=O)c3ccccc3C(=O)c2cc1C
InChI
1S/C16H12O2/c1-9-7-13-14(8-10(9)2)16(18)12-6-4-3-5-11(12)15(13)17/h3-8H,1-2H3
InChI key
KIJPZYXCIHZVGP-UHFFFAOYSA-N
General description
2,3-Dimethylanthraquinone is 2,3-dimethyl substituted anthraquinone and its synthesis has been reported. Electron transfer rate constants for the reaction between radical anions of 2,3-dimethylanthraquinone with benzyl bromide has been evaluated. The photoreactivity of 2,3-dimethylanthraquinone has been evaluated to investigate its usefulness as a photo-reagent for the analysis of ginsenosides using photoreduction fluorescence (PRF) detection method.
Application
2,3-Dimethylanthraquinone may be used as starting reagent in the regioselective synthesis of 6-amino-2,3-anthracenedimethanol.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
2, 3-Dimethylanthraquinone.
Allen CFH and Alan Bell.
Organic Syntheses, 37-37 (1942)
Regioselective syntheses of 5-and 6-aminoanthracene-2, 3-dimethanol.
Sun S and Desper J.
Tetrahedron, 54(3), 411-422 (1998)
Is samarium diiodide an inner-or outer-sphere electron donating agent?
Enem?rke RJ, et al.
Chemical Communications (Cambridge, England), 4, 343-344 (1999)
Photoreactivity of anthraquinones for the analysis of ginsenosides using photoreduction fluorescence detection-HPLC
Park MK, et al.
Archives of Pharmacal Research, 19(6), 562-565 (1996)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service