309982
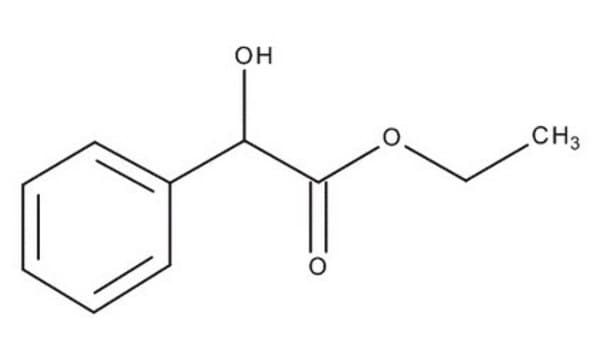
Ethyl (R)-(−)-mandelate
99%, optical purity ee: 99% (GLC)
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5CH(OH)CO2C2H5
CAS Number:
Molecular Weight:
180.20
MDL number:
UNSPSC Code:
12352108
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
optical activity
[α]21/D −134°, c = 3 in chloroform
optical purity
ee: 99% (GLC)
bp
103-105 °C/2 mmHg (lit.)
mp
33-34 °C (lit.)
functional group
ester
hydroxyl
phenyl
SMILES string
CCOC(=O)[C@H](O)c1ccccc1
InChI
1S/C10H12O3/c1-2-13-10(12)9(11)8-6-4-3-5-7-8/h3-7,9,11H,2H2,1H3/t9-/m1/s1
InChI key
SAXHIDRUJXPDOD-SECBINFHSA-N
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Asteriani R Dewanti et al.
Biochemistry, 43(7), 1883-1890 (2004-02-18)
(S)-Mandelate dehydrogenase (MDH) from Pseudomonas putida is a flavin mononucleotide (FMN)-dependent enzyme that oxidizes (S)-mandelate to benzoylformate. In this work, we show that the ethyl and methyl esters of (S)-mandelic acid are substrates for MDH. Although the binding affinity of
H Xiao et al.
Chinese journal of biotechnology, 9(1), 33-39 (1993-01-01)
Candida cyclindracea lipase (CCL) was added to "sodium dodecyl sulfonate (AS)/n-butanol/n-octane/n-octane" water-in-oil microemulsion to catalyze the hydrolysis of ethyl mandelate and the esterification of alpha-bromopropionic acid with n-butanol, respectively. The catalytic activity of CCL in the above microemulsions was higher
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service