161470
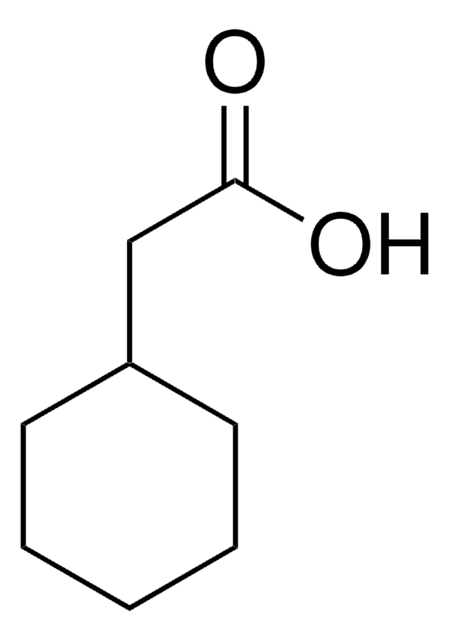
3-Cyclohexanepropionic acid
99%
Synonym(s):
3-Cyclohexylpropionic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H11CH2CH2CO2H
CAS Number:
Molecular Weight:
156.22
Beilstein:
2042799
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.464 (lit.)
bp
275.8 °C (lit.)
mp
14-17 °C (lit.)
density
0.912 g/mL at 25 °C (lit.)
SMILES string
OC(=O)CCC1CCCCC1
InChI
1S/C9H16O2/c10-9(11)7-6-8-4-2-1-3-5-8/h8H,1-7H2,(H,10,11)
InChI key
HJZLEGIHUQOJBA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
3-Cyclohexanepropionic acid can be used:
- As a starting material in the synthesis of 2-[(1,2,4-triazol-3-yl)thio]acetamide derivatives for in vitro paraoxonase-1 (PON1) studies.
- To synthesize chemical intermediates such as cyclohexanepropanenitrile , cyclohexanepropanamide , cyclohexanepropanol , and 1-oxaspiro[4.5]decan-2-one .
Biochem/physiol Actions
3-Cyclohexanepropionic acid facilitates oral delivery of cromolyn via permeation across/through the membrane in rats.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
266.0 °F
Flash Point(C)
130 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A Leone-Bay et al.
Pharmaceutical research, 13(2), 222-226 (1996-02-01)
Herein we report the discovery of a group of derivatized alpha-amino acids that increase the oral bioavailability of sodium cromolyn. We prepared three N-acylated alpha-amino acids and used these compounds to demonstrate the oral delivery of cromolyn in an in
Ethylenation of aldehydes to 3-propanal, propanol and propanoic acid derivatives
Payne DT, et al.
Scientific reports, 7(1), 1720-1720 (2017)
Continuous flow synthesis under high-temperature/high-pressure conditions using a resistively heated flow reactor
Adeyemi A, et al.
Organic Process Research & Development, 21(7), 947-955 (2017)
C-H oxygenation at tertiary carbon centers using iodine oxidant
Kiyokawa K, et al.
Chemical Communications (Cambridge, England), 54(55), 7609-7612 (2018)
Remote Allylation of Unactivated C (sp3)-H Bonds Triggered by Photogenerated Amidyl Radicals
Xu B and Tambar UK
ACS Catalysis, 21(7), 947-955 (2019)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service