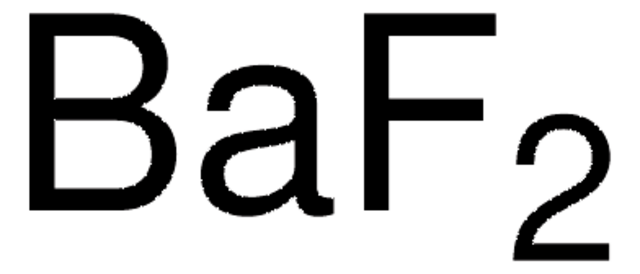All Photos(2)
About This Item
Empirical Formula (Hill Notation):
BaF2
CAS Number:
Molecular Weight:
175.32
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.55
grade:
for analytical purposes
form:
powder
Recommended Products
grade
for analytical purposes
Assay
98%
form
powder
bp
2260 °C (lit.)
density
4.89 g/mL at 25 °C (lit.)
SMILES string
F[Ba]F
InChI
1S/Ba.2FH/h;2*1H/q+2;;/p-2
InChI key
OYLGJCQECKOTOL-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
Related Categories
Features and Benefits
Primary glass modifier in fluorozirconate glasses.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Acute Tox. 4 Inhalation
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Refractive properties of barium fluoride.
Mayakova MN, et al.
Journal of the Optical Society of America, 54(5), 628-630 (1964)
Synthesis and fluorescence of neodymium-doped barium fluoride nanoparticles.
Bender CM, et al.
Chemistry of Materials, 12(7), 1969-1976 (2000)
Large barium fluoride detectors.
Wisshak K and Kappeler F.
Nuclear Instruments & Methods in Physics Research. Section A, Accelerators, Spectrometers, Detectors and Associated Equipment, 227(1), 91-96 (1984)
C W van Eijk et al.
Medical progress through technology, 17(3-4), 243-247 (1991-01-01)
A detector has been developed for position sensitive registration of annihilation radiation. The 511 keV gamma rays are detected in a set of BaF2 scintillation crystals coupled to low pressure TMAE-filled photosensitive multi wire chambers. In a first experimental detector
T J Bastow et al.
Solid state nuclear magnetic resonance, 6(1), 95-100 (1996-02-01)
The X-ray storage phosphor materials BaFC1 and BaFBr differ greatly in their 137Ba nuclear electric quadrupole interaction, despite a common crystal structure. The quadrupole coupling constant in BaFCl is relatively small and increases rapidly with temperature, in an unusual manner:
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service