All Photos(1)
About This Item
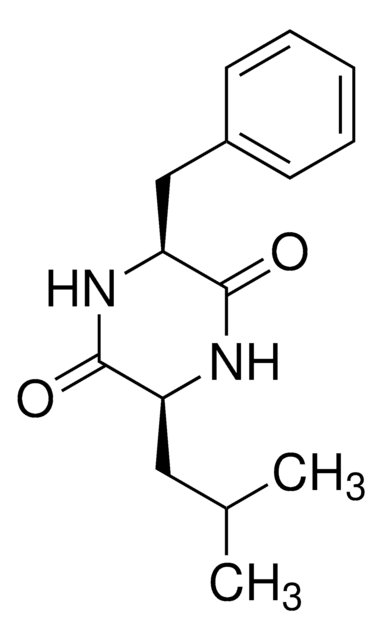
Empirical Formula (Hill Notation):
C12H16N2O3
CAS Number:
Molecular Weight:
236.27
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Product Name
Phe-Ala,
Assay
≥98.0% (TLC)
Quality Level
form
powder
color
white
storage temp.
−20°C
SMILES string
C[C@H](NC(=O)[C@@H](N)Cc1ccccc1)C(O)=O
InChI
1S/C12H16N2O3/c1-8(12(16)17)14-11(15)10(13)7-9-5-3-2-4-6-9/h2-6,8,10H,7,13H2,1H3,(H,14,15)(H,16,17)/t8-,10-/m0/s1
InChI key
MIDZLCFIAINOQN-WPRPVWTQSA-N
Gene Information
human ... SLC15A1(6564)
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
F Döring et al.
Biochemical and biophysical research communications, 232(3), 656-662 (1997-03-27)
The methylotrophic yeast Pichia pastoris was used for heterologous expression of the rabbit intestinal peptide transporter PepT1 and its functional characterization. PepT1 mediates the electrogenic transmembrane transport of di- and tripeptides and peptido-mimetics such as beta-lactam antibiotics and ACE-inhibitors. Functional
Shu-Hui Chen et al.
Bioorganic & medicinal chemistry letters, 14(1), 245-250 (2003-12-20)
With the aim of reducing molecular weight and adjusting log D value of BACE inhibitors to more favorable range for BBB penetration and better bioavailability, we synthesized and evaluated several series of P3 cap modified BACE inhibitors obtained via replacement
I Knütter et al.
Biochemistry, 40(14), 4454-4458 (2001-04-04)
This study was initiated to develop inhibitors of the intestinal H(+)/peptide symporter. We provide evidence that the dipeptide derivative Lys[Z(NO(2))]-Pro is an effective competitive inhibitor of mammalian PEPT1 with an apparent binding affinity of 5-10 microM. Characterization of the interaction
D Meredith et al.
The American journal of physiology, 269(2 Pt 1), L137-L143 (1995-08-01)
The transport of a hydrolysis-resistant dipeptide, D-phenylalanyl-L-alanine (D-Phe-L-Ala), has been studied by high-performance liquid chromatography in rat lung epithelial cells and apical membrane vesicles. Time-dependent uptake of D-Phe-L-Ala into isolated type II pneumocytes was shown. Uptake was saturable, and Michaelis-Menten
C H Görbitz
Acta crystallographica. Section C, Crystal structure communications, 57(Pt 5), 575-576 (2001-05-16)
A new type of molecular arrangement for dipeptides is observed in the crystal structure of L-phenylalanyl-L-alanine dihydrate, C12H16N2O3-2H2O. Two L-Phe and two L-Ala side chains aggregate into large hydrophobic columns within a three-dimensional hydrogen-bond network.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service