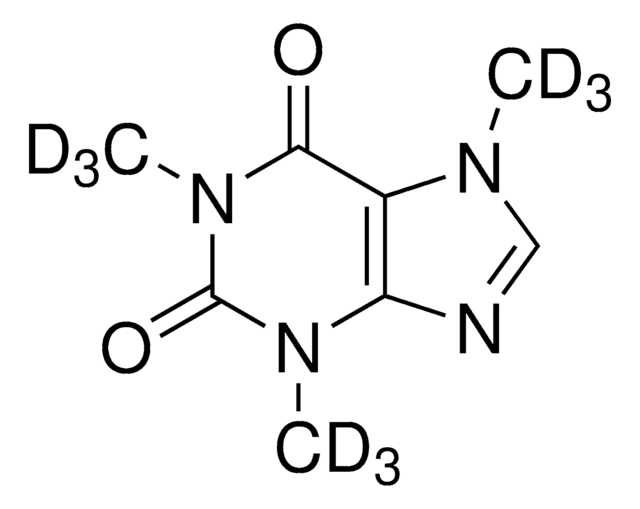
C-082
Caffeine-13C3 solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®
Synonym(s):
Caffeine-trimethyl-13C3 solution
About This Item
Recommended Products
grade
certified reference material
Quality Level
form
liquid
feature
SNAP-N-SPIKE®, SNAP-N-SHOOT®
packaging
ampule of 1 mL
manufacturer/tradename
Cerilliant®
concentration
1.0 mg/mL in methanol
technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
application(s)
forensics and toxicology
format
single component solution
storage temp.
−20°C
SMILES string
[13CH3]N1C(=O)N([13CH3])c2ncn([13CH3])c2C1=O
InChI
1S/C8H10N4O2/c1-10-4-9-6-5(10)7(13)12(3)8(14)11(6)2/h4H,1-3H3/i1+1,2+1,3+1
InChI key
RYYVLZVUVIJVGH-VMIGTVKRSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Caffeine-¹³C₃ isotopic standard for mass spectrometry: Recent research in "Physical Chemistry Chemical Physics" leverages the stable isotopic properties of Caffeine-¹³C₃ to explore the photo-deactivation processes of isothiazolinones, highlighting its utility in tracing environmental contaminants through advanced non-adiabatic dynamics investigations (de Araújo et al., 2024).
- Stable isotope-labeled caffeine for metabolic studies: "ACS Applied Materials & Interfaces" features a study employing Caffeine-¹³C₃ to enhance the sensitivity of terahertz detection in monolayer materials, demonstrating its application in precise quantum sensing and material characterization (Wang and Yu, 2024).
- ¹³C-labeled caffeine solution for pharmaceutical research: "JMIR Medical Education" utilized Caffeine-¹³C₃ in biostatistics education through interactive simulations, indicating its significance in educational frameworks for pharmaceutical research, particularly in statistical data analysis and interpretation (Thiesmeier and Orsini, 2024).
- High-purity ¹³C₃ caffeine analytical reference material: Research published in "The Journal of Physical Chemistry Letters" explores the proximity-induced exchange interactions using Caffeine-¹³C₃, paving new pathways for quantum sensing technologies by utilizing stable isotopic labels in experimental setups (Shen et al., 2024).
- Caffeine-¹³C₃ for bioanalytical method development: "Nano Letters" discusses the use of Caffeine-¹³C₃ in studying the topology-engineered orbital Hall effect in two-dimensional ferromagnets, illustrating its role in cutting-edge nanotechnology research and materials science (Chen et al., 2024).
Legal Information
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
Target Organs
Eyes
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
49.5 °F - closed cup
Flash Point(C)
9.7 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service