730300
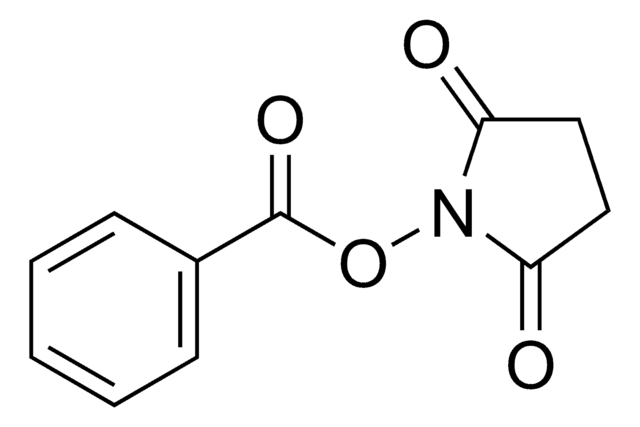
Methacrylic acid N-hydroxysuccinimide ester
98%
Synonym(s):
N-(Methacryloxy)succinimide, N-(Methacryloyloxy)succinimide
About This Item
Recommended Products
Assay
98%
form
solid
mp
101-105 °C
storage temp.
2-8°C
SMILES string
CC(=C)C(=O)ON1C(=O)CCC1=O
InChI
1S/C8H9NO4/c1-5(2)8(12)13-9-6(10)3-4-7(9)11/h1,3-4H2,2H3
InChI key
ACGJEMXWUYWELU-UHFFFAOYSA-N
General description
Application
- As a monomer to prepare degradable amphiphilic diblock copolymer microparticles via RAFT polymerization, for low pH-triggered drug delivery. NHS-MA can shield the drug molecule from degradation, enhance its solubility, and improve its pharmacokinetic properties.
- For the surface functionalization of poly-ε-caprolactone (PCL) scaffolds used for tissue engineering. NHS groups are used to couple with chitosan of various molecular weights.
- To prepare biocompatible polymer hydrogel for enzymatic biofuel cells. The hydrogel can serve as an enzyme-immobilizing matrix for enzymatic bioelectrodes.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service