SAE0172
Mpro, 3CL Protease from coronavirus SARS-COV-2
recombinant protein, lyophilized product
Synonym(s):
2019-nCoV, 3C Like proteinase, 3C-like main protease, 3CL Mpro, 3CL protease, 3CL proteinase, 3CLpro, COVID-19, COVID-2019, SARS-CoV-2, coronavirus
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
UNSPSC Code:
12352204
NACRES:
NA.54
Recommended Products
General description
Mpro, 3CL is a critical enzyme for the virus life cycle. It activity yield non-structural proteins that are crucial for genome replication and Coronavirus virion production: RNA-dependent RNA polymerase, a helicase, ribonucleases and 3CLpro itself, from two types of polyproteins (pp1a and pp1ab).The maturation of SARS-Cov2 virus (SARS coronavirus) depends on cleavage of the overlapping large polyproteins 1a and 1ab by two viral proteases:
SARS-CoV Mpro, 3CL exists as a homodimer and each protomer has an active site. The proteolytic cleavage of 1ab polyprotein by Mpro occurs at 11 sites: 7 sites within the 1a polyprotein, and 11 sites within the 1ab polyprotein. This results in maturation of 16 viral non-structural proteins.Mpro protease forms a functional homodimer. Both the N-terminus and the C- terminus of Mpro have been shown to be critical for dimer formation and for enzyme function.
The Mpro protease, 3CLpro is an ideal target for antiviral drug design due to its high conservation between different coronavirus strains and absence of functional analogs in the human proteome. Mpro protease from SARS-CoV1 and SARS-CoV2 are functionally identical.
- Mpro (main protease)
- PLpro (Papain-like protease)
SARS-CoV Mpro, 3CL exists as a homodimer and each protomer has an active site. The proteolytic cleavage of 1ab polyprotein by Mpro occurs at 11 sites: 7 sites within the 1a polyprotein, and 11 sites within the 1ab polyprotein. This results in maturation of 16 viral non-structural proteins.Mpro protease forms a functional homodimer. Both the N-terminus and the C- terminus of Mpro have been shown to be critical for dimer formation and for enzyme function.
The Mpro protease, 3CLpro is an ideal target for antiviral drug design due to its high conservation between different coronavirus strains and absence of functional analogs in the human proteome. Mpro protease from SARS-CoV1 and SARS-CoV2 are functionally identical.
Features and Benefits
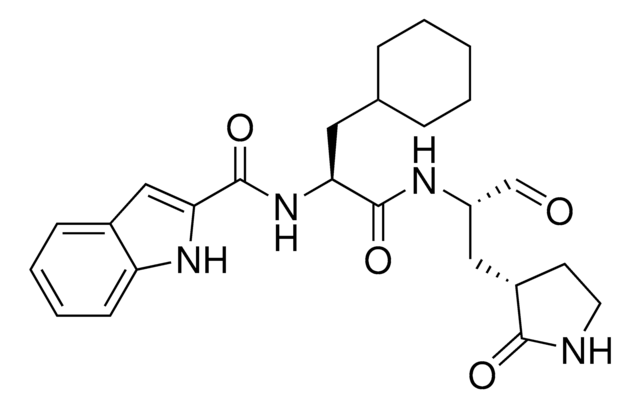
Mpro, 3CL Protease (3C-like protease)is the main protease of Human Coronavirus SARS-CoV-2. Mpro 3CL Protease is a cysteine protease. Mpro protease cleaves proteins with sequences including LQ[S/A/G) c-terminal to the glutamine residue.
Physical form
The product is supplied lyophilized from 20 mM HEPES, 2.5% Trehalose, and 0.05% Tween 20
This product contains the complete sequence of Mpro protease (Accession: YP_009725301.1) without any additional tags. The product is supplied lyophilized from 20 mM HEPES, 2.5% Trehalose, and 0.05% Tween 20.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Bhupesh Goyal et al.
ACS combinatorial science, 22(6), 297-305 (2020-05-14)
A new coronavirus (CoV) caused a pandemic named COVID-19, which has become a global health care emergency in the present time. The virus is referred to as SARS-CoV-2 (severe acute respiratory syndrome-coronavirus-2) and has a genome similar (∼82%) to that
Huifang Li et al.
Journal of agricultural and food chemistry, 69(41), 12197-12208 (2021-09-30)
Severe acute respiratory syndrome coronavirus (SARS-CoV-2) main protease (Mpro) inhibitors are considered as potential treatments for coronavirus disease 2019, and dietary polyphenols show promise in SARS-CoV-2 Mpro inhibition based on in silico studies. In the present study, we utilize a
Linlin Zhang et al.
Science (New York, N.Y.), 368(6489), 409-412 (2020-03-22)
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) is a global health emergency. An attractive drug target among coronaviruses is the main protease (Mpro, also called 3CLpro) because of its essential role in processing
Keqiang Fan et al.
The Journal of biological chemistry, 279(3), 1637-1642 (2003-10-17)
The 3C-like proteinase of severe acute respiratory syndrome (SARS) coronavirus has been proposed to be a key target for structural-based drug design against SARS. In order to understand the active form and the substrate specificity of the enzyme, we have
Haitao Yang et al.
Current pharmaceutical design, 12(35), 4573-4590 (2006-12-16)
Coronaviruses (CoVs), a genus containing about 26 known species to date, cause highly prevalent diseases and are often severe or fatal in humans and animals. In 2003, a previously unknown coronavirus was identified to be the etiological agent of a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service