I9781
Importin β1 human
≥80% (SDS-PAGE), recombinant, expressed in E. coli (N-terminal histidine tagged), buffered aqueous glycerol solution
Synonym(s):
Importin β, Karyopherin β1, p97
Sign Into View Organizational & Contract Pricing
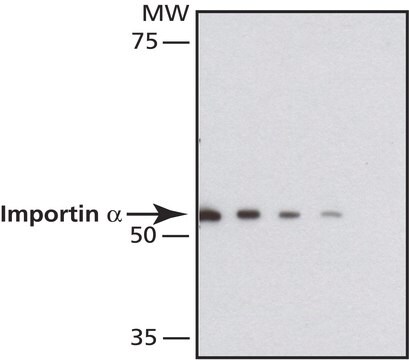
All Photos(1)
About This Item
Recommended Products
recombinant
expressed in E. coli (N-terminal histidine tagged)
Assay
≥80% (SDS-PAGE)
form
buffered aqueous glycerol solution
mol wt
~97 kDa by SDS-PAGE
UniProt accession no.
shipped in
dry ice
storage temp.
−70°C
Gene Information
human ... KPNB1(3837)
General description
Importin β1 has 19 tandemly repeated HEAT (huntingtin, elongation factor 3 (EF3), protein phosphatase 2A (PP2A) and the yeast kinase TOR1) motifs. The gene is mapped to human chromosome 17q21. The protein shuttles between the cytoplasm and nucleus. It belongs to the importin β family of proteins.
Biochem/physiol Actions
Importin β, an import receptor, is a prototype of the nuclear transport receptor family, which comprises in human more than 20 proteins (90-180 kDa). These proteins interact directly with nuclear pore complex (NPC) and mediate nucleocytoplasmic transport. Importin β imports into the nucleus proteins carrying canonical nuclear localization signals (NLS) as well as UsnRNAs, which it binds via adaptor proteins, importin α and snurpotin-1 respectively. Importin β also binds directly, without adaptors, ribosomal proteins, Smad proteins, and virus derived proteins such as HIV Rev and Tat, that contain non classical NLS. In addition, Improtin b has been shown to be involved in the nuclear envelope assembly process.
The mechanism of importin β action in nuclear import can be demonstrated by the well-studied import of proteins containing classical NLS. Importin β forms a complex with Importin α, which, in turn, binds the cargo protein via its NLS. The Impβ/Impα/cargo complex translocates into the nucleus. When the complex reaches the nuclear site of the NPC, Ran-GTP binds the Impβ to form Impβ/Ran-GTP complex and released the Impα and the cargo protein. The Impβ/Ran-GTP complex is then exported to the cytoplasm where the complex dissociates upon hydrolysis of GTP to GDP, making Impβ ready for a new import cycle.
The mechanism of importin β action in nuclear import can be demonstrated by the well-studied import of proteins containing classical NLS. Importin β forms a complex with Importin α, which, in turn, binds the cargo protein via its NLS. The Impβ/Impα/cargo complex translocates into the nucleus. When the complex reaches the nuclear site of the NPC, Ran-GTP binds the Impβ to form Impβ/Ran-GTP complex and released the Impα and the cargo protein. The Impβ/Ran-GTP complex is then exported to the cytoplasm where the complex dissociates upon hydrolysis of GTP to GDP, making Impβ ready for a new import cycle.
Physical form
Solution in 5% glycerol containing 20 mM Hepes, pH 7.5, 110 mM potassium-acetate, 2 mM magnesium-acetate, 0.5 mM EGTA, 0.1 mM ATP, 2 mM DTT, and protease inhibitors.
Storage Class Code
12 - Non Combustible Liquids
WGK
nwg
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Qian An et al.
International journal of cancer, 103(2), 194-204 (2002-11-28)
Suppression subtractive hybridization (SSH) was applied to identify differentially expressed genes in the SV40LT immortalized human bronchial epithelial cell line Y-BE, with normal human bronchial epithelial cells (HBEC) as a control. Two cDNA libraries of up- and downregulated genes were
G Cingolani et al.
Nature, 399(6733), 221-229 (1999-06-03)
Cytosolic proteins bearing a classical nuclear localization signal enter the nucleus bound to a heterodimer of importin-alpha and importin-beta (also called karyopherin-alpha and -beta). The formation of this heterodimer involves the importin-beta-binding (IBB) domain of importin-alpha, a highly basic amino-terminal
Amnon Harel et al.
Molecular cell, 16(3), 319-330 (2004-11-05)
Importin beta, once thought to be exclusively a nuclear transport receptor, is emerging as a global regulator of diverse cellular functions. Importin beta acts positively in multiple interphase roles: in nuclear import, as a chaperone for highly charged nuclear proteins
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service