All Photos(2)
About This Item
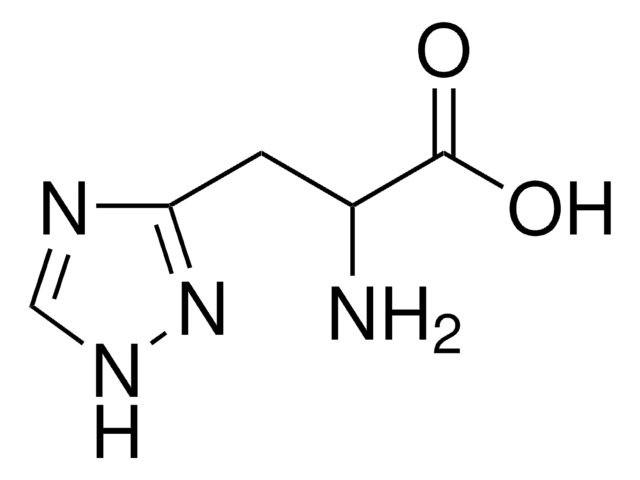
Empirical Formula (Hill Notation):
C10H11N3O2 · H2O
CAS Number:
Molecular Weight:
223.23
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Product Name
DL-7-Azatryptophan hydrate,
Assay
≥98% (TLC)
form
powder
color
white to off-white
storage temp.
−20°C
SMILES string
O.NC(Cc1c[nH]c2ncccc12)C(O)=O
InChI
1S/C10H11N3O2.H2O/c11-8(10(14)15)4-6-5-13-9-7(6)2-1-3-12-9;/h1-3,5,8H,4,11H2,(H,12,13)(H,14,15);1H2
InChI key
PXDRHYQAIUZKHN-UHFFFAOYSA-N
Biochem/physiol Actions
DL-7-Azatryptophan is a racemic mixture of D- and L-7-azatryptophan which together with L-tryptophan is a synergistic inducer of tryptophan oxygenase of Pseudomonas acidovorans. DL-7-Azatryptophan inhibits photosynthetic carbon assimilation, photosynthetic oxygen evolution and nitrogen metabolism in Anabaena sp. Strain 1F, a marine filamentous, heterocystous cyanobacterium.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
H Rosenfeld et al.
Journal of bacteriology, 97(2), 697-704 (1969-02-01)
The process of induction of tryptophan oxygenase in Pseudomonas acidovorans is typical of many microbial enzyme induction systems, in that it (i) requires cell multiplication and de novo protein synthesis, (ii) is subject to catabolite repression, (iii) results in the
P Cioni et al.
Biochemical and biophysical research communications, 248(2), 347-351 (1998-07-24)
The tryptophan analogues 7-azaindole (7-Aza W) and 5-hydroxytryptophan (5-OH W) have a significant absorbance between 310-320 nm, which allows them to act as selective luminescence probes in protein mixtures containing a large number of tryptophan residues. To assess the potential
Fernando Formaggio et al.
Advances in experimental medicine and biology, 527, 731-737 (2004-06-23)
Aal and 7-Atrp, quasi-isosteric with Trp, have been inserted together with a TOAC residue in two 3(10)-helical, model hexapeptides. The interaction of photoexcited AA1 and 7-Atrp with the nitroxide group of TOAC was investigated by time resolved EPR. Both peptides
P Soumillion et al.
Protein engineering, 8(5), 451-456 (1995-05-01)
The phage lambda lysozyme (lambda L) contains four tryptophans. These have been efficiently replaced by 7-azatryptophan (7aW) through biosynthetic incorporation into the overexpressed protein. Comparative analysis of the effect of temperature or pH on the fluorescence of the wild-type lambda
Julie M G Rogers et al.
Analytical biochemistry, 399(2), 182-189 (2009-12-29)
Fluorescence resonance energy transfer (FRET) provides a powerful means to study protein conformational changes. However, the incorporation of an exogenous FRET pair into a protein could lead to undesirable structural perturbations of the native fold. One of the viable strategies
Chromatograms
application for HPLCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service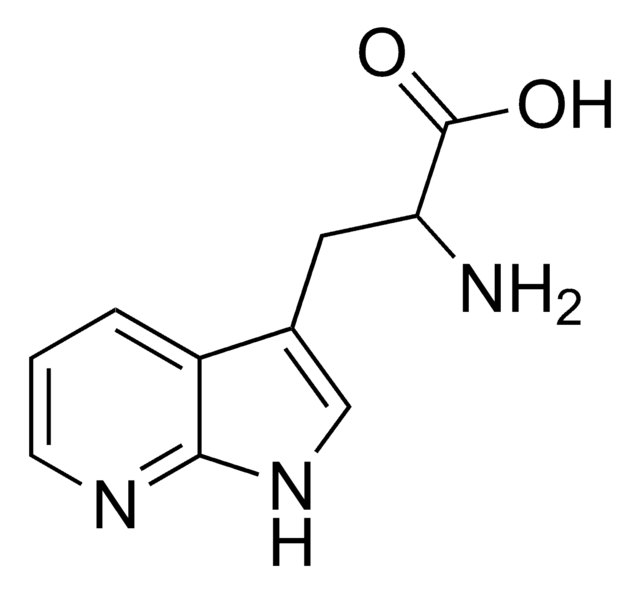



![1,2,3,4-Tetrahydro-9H-pyrido[3,4-b]indole 98%](/deepweb/assets/sigmaaldrich/product/structures/181/460/3d58bc34-1b5c-4295-bbac-3b52085670e8/640/3d58bc34-1b5c-4295-bbac-3b52085670e8.png)