All Photos(2)
About This Item
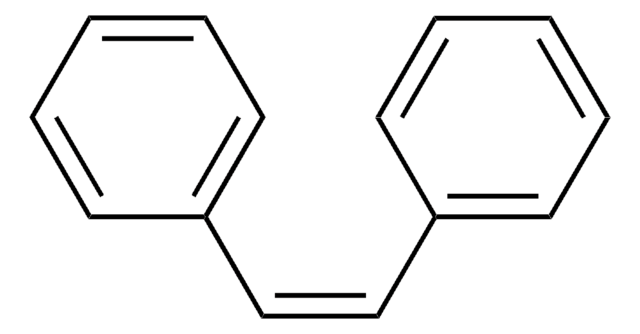
Linear Formula:
C6H5C≡CC6H5
CAS Number:
Molecular Weight:
178.23
Beilstein:
606478
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
crystals
bp
170 °C/19 mmHg (lit.)
mp
59-61 °C (lit.)
density
0.99 g/mL at 25 °C (lit.)
SMILES string
c1ccc(cc1)C#Cc2ccccc2
InChI
1S/C14H10/c1-3-7-13(8-4-1)11-12-14-9-5-2-6-10-14/h1-10H
InChI key
JRXXLCKWQFKACW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Neola F McKinley et al.
The Journal of organic chemistry, 71(25), 9552-9555 (2006-12-02)
Carbolithiation of diphenylacetylene can be exploited to generate (E)-1-lithio-1,2-diphenylalkyl-1-enes which can be reacted in situ with triisopropylborate to stereoselectively provide (E)-1,2-diphenyl-1-alkylene boronic acids. These tetrasubstituted vinylboronic acids served as versatile intermediates for the generation of tetrasubstituted olefins with retention of
Anion-dependent switching: dynamically controlling the conformation of hydrogen-bonded diphenylacetylenes.
Ian M Jones et al.
Angewandte Chemie (International ed. in English), 50(20), 4597-4600 (2011-04-16)
H Zhang et al.
Organic letters, 3(20), 3083-3086 (2001-09-28)
[reaction: see text] A variety of substituted beta- and gamma-carbolines have been prepared in good to excellent yields by the annulation of internal acetylenes by the tert-butylimines of N-substituted 3-iodoindole-2-carboxaldehydes and 2-haloindole-3-carboxaldehydes, respectively, in the presence of a palladium catalyst.
Ian M Jones et al.
Angewandte Chemie (International ed. in English), 50(52), 12569-12571 (2011-12-17)
The conformational equilibrium of a pH-dependent switch based on an intramolecularly H-bonded diphenylacetylene can be predictably biased by using electron-donating or -withdrawing groups. Furthermore, protonation of the electron-donating dimethylamino group converts it into an electron-withdrawing dimethylammonium cation with a concomitant
Kazuki Tainaka et al.
The journal of physical chemistry. B, 114(45), 14657-14663 (2010-06-01)
DNA-mediated charge transfer has recently received a substantial attention because of its biological relevance in the DNA damage and DNA repair as well as the potential applications to nanoscale electronic devices. In contrast to the numerous mechanistic studies on oxidative
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![4-[(4-Fluorophenyl)ethynyl]phenol AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/188/684/0b16c024-0d26-4b43-a607-60b40446e593/640/0b16c024-0d26-4b43-a607-60b40446e593.png)