659851
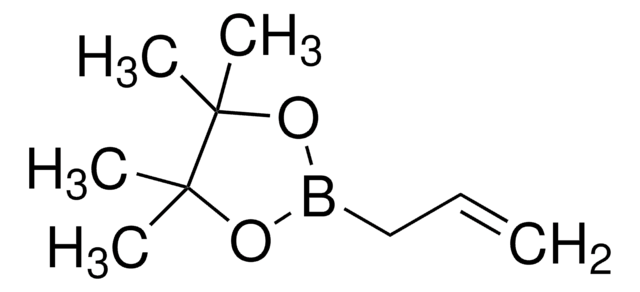
Cyclopropylboronic acid pinacol ester
96%
Synonym(s):
2-Cyclopropyl-4,4,5,5-tetramethyl-1,3,2-dioxaborolane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H17BO2
CAS Number:
Molecular Weight:
168.04
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
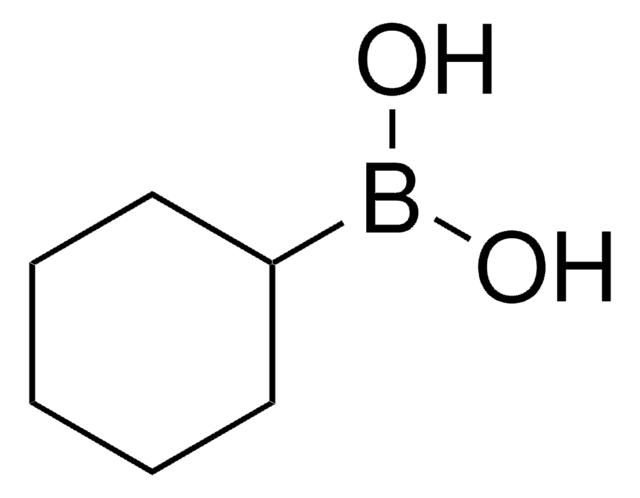
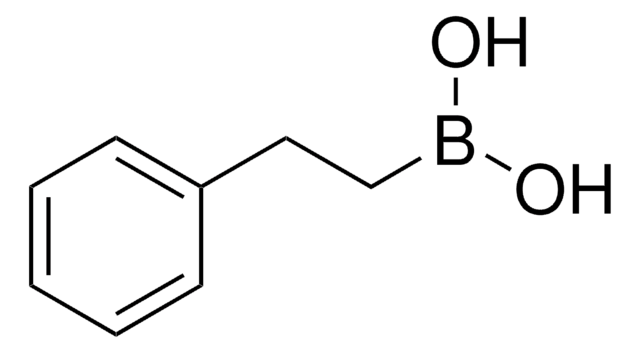
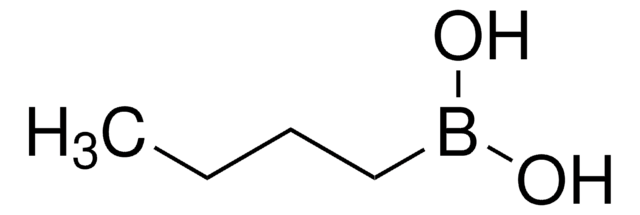
Recommended Products
Assay
96%
refractive index
n20/D 1.433
bp
146 °C
density
0.922 g/mL at 25 °C
SMILES string
CC1(C)OB(OC1(C)C)C2CC2
InChI
1S/C9H17BO2/c1-8(2)9(3,4)12-10(11-8)7-5-6-7/h7H,5-6H2,1-4H3
InChI key
XGBMQBPLWXTEPM-UHFFFAOYSA-N
Application
Cyclopropylboronic acid pinacol ester can be used:
- As a cyclopropylating reagent in the study of thiophenol S-cyclopropylation, catalyzed by copper(II) acetate.
- In one of the key synthetic steps for the preparation of 5-lipoxygenase activating protein (FLAP) inhibitor.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
104.0 °F
Flash Point(C)
40 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Emeline Benoit et al.
Beilstein journal of organic chemistry, 15, 1162-1171 (2019-07-12)
The copper-promoted S-cyclopropylation of thiophenols using cyclopropylboronic acid is reported. The procedure operates under simple conditions to afford the corresponding aryl cyclopropyl sulfides in moderate to excellent yields. The reaction tolerates substitution in ortho-, meta- and para-substitution as well as
Keith R Fandrick et al.
The Journal of organic chemistry, 80(3), 1651-1660 (2015-01-07)
A practical sequence involving a noncryogenic stereospecific boronate rearrangement followed by a robust formylation with an in situ generated DCM anion has been developed for the asymmetric construction of an all-carbon quaternary stereogenic center of a FLAP inhibitor. The key
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service