404810
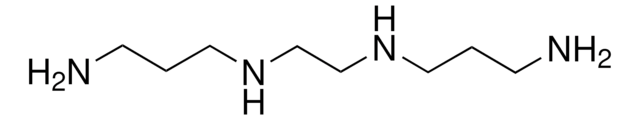
N,N′-Bis(3-aminopropyl)-1,3-propanediamine
technical grade, 90%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
H2N(CH2)3NH(CH2)3NH(CH2)3NH2
CAS Number:
Molecular Weight:
188.31
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Assay
90%
form
liquid
refractive index
n20/D 1.491 (lit.)
bp
98-103 °C/1 mmHg (lit.)
density
0.92 g/mL at 25 °C (lit.)
functional group
amine
SMILES string
NCCCNCCCNCCCN
InChI
1S/C9H24N4/c10-4-1-6-12-8-3-9-13-7-2-5-11/h12-13H,1-11H2
InChI key
ZAXCZCOUDLENMH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
N,N′-Bis(3-aminopropyl)-1,3-propanediamine, a linear polyamine, has been identified in white shrimp Penaeus setiferus by gas chromatography-mass spectrometry.
Application
N,N′-Bis(3-aminopropyl)-1,3-propanediamine (bappn) may be used in the preparation of [Ni(bappn)(ttcH)]·5H2O complex (ttch= trithiocyanurate dianion).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Anna Gasowska et al.
Journal of inorganic biochemistry, 101(10), 1362-1369 (2007-07-04)
Complexation reactions in the quaternary system Cu/ATP/3,3,3-tet/Urd have been studied. The stability constants of the complexes of the Cu(ATP)(3,3,3-tet)H(x)(Urd) type have been determined by computer analysis of the potentiometric titration. On the basis of the results of spectroscopic as well
Sandra Fouace et al.
Nucleic acids research, 32(1), 151-157 (2004-01-06)
Site-selective scission of ribonucleic acids (RNAs) has attracted considerable interest, since RNA is an intermediate in gene expression and the genetic material of many pathogenic viruses. Polyamine-imidazole conjugates for site-selective RNA scission, without free imidazole, were synthesized and tested on
D Leroy et al.
Biochemistry, 36(6), 1242-1250 (1997-02-11)
Protein kinase CK2 is a ubiquitous eukaryotic Ser/Thr kinase whose catalytic activity is enhanced several times by polyamines. We have shown previously that the regulatory beta-subunit of CK2 bears a polyamine binding site located in the region Asp51-Tyr110. In the
R H Hu et al.
The Biochemical journal, 328 ( Pt 1), 307-316 (1998-01-10)
Treatment of Chinese hamster ovary cells with alpha-difluoromethylornithine for 3 days, followed by exposure to cycloheximide, led to an unregulated, rapid and massive accumulation of polyamine analogues. This accumulation led to cell death by apoptosis within a few hours. Clear
Anurupa Shrestha et al.
Bioorganic & medicinal chemistry letters, 19(9), 2478-2481 (2009-04-01)
We have previously shown that simple N-acyl or N-alkyl polyamines bind to and sequester Gram-negative bacterial lipopolysaccharide, affording protection against lethality in animal models of endotoxicosis. Several iterative design-and-test cycles of SAR studies, including high-throughput screens, had converged on compounds
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service